Belzutifan for von Hippel-Lindau Disease: Pancreatic Lesion Population of the Phase 2 LITESPARK-004 Study
- PMID: 38393723
- PMCID: PMC11061599
- DOI: 10.1158/1078-0432.CCR-23-2592
Belzutifan for von Hippel-Lindau Disease: Pancreatic Lesion Population of the Phase 2 LITESPARK-004 Study
Abstract
Purpose: Primary analysis of the ongoing, single-arm, phase 2 LITESPARK-004 study (NCT03401788) showed clinically meaningful antitumor activity in von Hippel-Lindau (VHL) disease-associated renal cell carcinoma (RCC) and other neoplasms with belzutifan treatment. We describe results of belzutifan treatment for VHL disease-associated pancreatic lesions [pancreatic neuroendocrine tumors (pNET) and serous cystadenomas].
Patients and methods: Adults with VHL diagnosis based on germline VHL alteration, ≥1 measurable RCC tumor, no renal tumor >3 cm or other VHL neoplasm requiring immediate surgery, Eastern Cooperative Oncology Group performance status of 0 or 1, and no prior systemic anticancer treatment received belzutifan 120 mg once daily. End points included objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), and linear growth rate (LGR) in all pancreatic lesions and pNETs per RECIST version 1.1 by independent review committee, and safety.
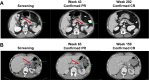
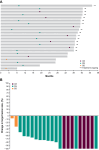
Results: All 61 enrolled patients (100%) had ≥1 pancreatic lesion and 22 (36%) had ≥1 pNET measurable at baseline. Median follow-up was 37.8 months (range, 36.1-46.1). ORR was 84% [51/61; 17 complete responses (CR)] in pancreatic lesions and 91% (20/22; 7 CRs) in pNETs. Median DOR and median PFS were not reached in pancreatic lesions or pNETs. After starting treatment, median LGR for pNETs was -4.2 mm per year (range, -7.9 to -0.8). Eleven patients (18%) had ≥1 grade 3 treatment-related adverse event (AE). No grade 4 or 5 treatment-related AEs occurred.
Conclusions: Belzutifan continued to show robust activity and manageable safety in VHL disease-associated pNETs.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Ganeshan D, Menias CO, Pickhardt PJ, Sandrasegaran K, Lubner MG, Ramalingam P, et al. . Tumors in von Hippel–Lindau syndrome: from head to toe-comprehensive state-of-the-art review. Radiographics 2018;38:849–66. - PubMed
-
- Choueiri TK, Kaelin WG. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat Med 2020;26:1519–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

