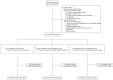
Halofuginone for non-hospitalized adult patients with COVID-19 a multicenter, randomized placebo-controlled phase 2 trial. The HALOS trial
- PMID: 38394069
- PMCID: PMC10889621
- DOI: 10.1371/journal.pone.0299197
Halofuginone for non-hospitalized adult patients with COVID-19 a multicenter, randomized placebo-controlled phase 2 trial. The HALOS trial
Abstract
Background: Halofuginone (PJS-539) is an oral prolyl-tRNA synthetase inhibitor that has a potent in vitro activity against SARS-CoV-2 virus. The safety and efficacy of halofuginone in Covid-19 patients has not been studied.
Methods: We conducted a phase II, randomized, double-blind, placebo-controlled, dose ranging, safety and tolerability trial of halofuginone in symptomatic (≤ 7 days), mostly vaccinated, non-hospitalized adults with mild to moderate Covid-19. Patients were randomized in a 1:1:1 ratio to receive halofuginone 0.5mg, 1mg or placebo orally once daily for 10 days. The primary outcome was the decay rate of the SARS-CoV-2 viral load logarithmic curve within 10 days after randomization.
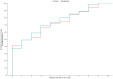
Results: From September 25, 2021, to February 3, 2022, 153 patients were randomized. The mean decay rate in SARS-CoV-2 viral load log10 within 10 days was -3.75 (95% CI, -4.11; -3.19) in the placebo group, -3.83 (95% CI, -4.40; -2.27) in the halofuginone 0.5mg group and -4.13 (95% CI, -4.69; -3.57) in the halofuginone 1mg group, with no statistically significant difference in between placebo vs. halofuginone 0.5mg (mean difference -0.08; 95% CI -0.82 to 0.66, p = 0.96) and between placebo vs. halofuginone 1mg (mean difference -0.38; 95% CI, -1.11; 0.36, p = 0.41). There was no difference on bleeding episodes or serious adverse events at 28 days.
Conclusions: Among non-hospitalized adults with mild to moderate Covid-19 halofuginone treatment was safe and well tolerated but did not decrease SARS-CoV-2 viral load decay rate within 10 days.
Copyright: © 2024 Tomazini et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
S.T is a co-inventor and receives royalties from patents owned by University of California San Diego (UCSD) and is a co-founder and has an equity interest in Oxitope, LLC and its affiliates, Kleanthi Diagnostics, LLC and Covicept Therapeutics, Inc and has a dual appointment at UCSD and Ionis Pharmaceuticals; A.F.C has received contract payments from Nurix Therapeutics, Inc. and has options in Covicept Therapeutics; J.D.E is a cofounder, consultant and shareholder in Covicept Therapeutics (San Diego, CA) and TEGA Therapeutics (La Jolla, CA); P.L.S.M.G. is a cofounder, consultant and shareholder in Covicept Therapeutics (San Diego, CA). All remaining authors: No conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials, although some restrictions might apply as stated in the submission.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous