Antiviral Protein-Protein Interaction Inhibitors
- PMID: 38394369
- PMCID: PMC10945500
- DOI: 10.1021/acs.jmedchem.3c01543
Antiviral Protein-Protein Interaction Inhibitors
Abstract
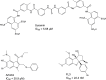
Continually repeating outbreaks of pathogenic viruses necessitate the construction of effective antiviral strategies. Therefore, the development of new specific antiviral drugs in a well-established and efficient manner is crucial. Taking into account the strong ability of viruses to change, therapies with diversified molecular targets must be sought. In addition to the widely explored viral enzyme inhibitor approach, inhibition of protein-protein interactions is a very valuable strategy. In this Perspective, protein-protein interaction inhibitors targeting HIV, SARS-CoV-2, HCV, Ebola, Dengue, and Chikungunya viruses are reviewed and discussed. Antibodies, peptides/peptidomimetics, and small molecules constitute three classes of compounds that have been explored, and each of them has some advantages and disadvantages for drug development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses.J Virol. 2016 Oct 14;90(21):9683-9692. doi: 10.1128/JVI.01347-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535047 Free PMC article.
-
Molecular Docking and In-Silico Analysis of Natural Biomolecules against Dengue, Ebola, Zika, SARS-CoV-2 Variants of Concern and Monkeypox Virus.Int J Mol Sci. 2022 Sep 22;23(19):11131. doi: 10.3390/ijms231911131. Int J Mol Sci. 2022. PMID: 36232431 Free PMC article.
-
Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses.J Virol. 2019 Apr 3;93(8):e02185-18. doi: 10.1128/JVI.02185-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700611 Free PMC article.
-
The Drug Targets and Antiviral Molecules for Treatment of Ebola Virus Infection.Curr Top Med Chem. 2017;17(3):361-370. doi: 10.2174/1568026616666160829161318. Curr Top Med Chem. 2017. PMID: 27572081 Review.
-
Small molecule inhibitors of ebola virus infection.Drug Discov Today. 2015 Feb;20(2):277-86. doi: 10.1016/j.drudis.2014.12.010. Epub 2014 Dec 19. Drug Discov Today. 2015. PMID: 25532798 Review.
Cited by
-
New insights into protein-protein interaction modulators in drug discovery and therapeutic advance.Signal Transduct Target Ther. 2024 Dec 6;9(1):341. doi: 10.1038/s41392-024-02036-3. Signal Transduct Target Ther. 2024. PMID: 39638817 Free PMC article. Review.
-
Mass Spectrometry-Based Proteomics Technologies to Define Endogenous Protein-Protein Interactions and Their Applications to Cancer and Viral Infectious Diseases.Mass Spectrom Rev. 2025 Feb 9:10.1002/mas.21926. doi: 10.1002/mas.21926. Online ahead of print. Mass Spectrom Rev. 2025. PMID: 39924651 Free PMC article. Review.
-
Molecular Mechanism of pH-Induced Protrusion Configuration Switching in Piscine Betanodavirus Implies a Novel Antiviral Strategy.ACS Infect Dis. 2024 Sep 13;10(9):3304-3319. doi: 10.1021/acsinfecdis.4c00407. Epub 2024 Aug 1. ACS Infect Dis. 2024. PMID: 39087906 Free PMC article.
-
SPIKENET: An Evidence-Based Therapy for Long COVID.Viruses. 2024 May 24;16(6):838. doi: 10.3390/v16060838. Viruses. 2024. PMID: 38932130 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Miscellaneous

