Clinical Characteristics and Long-Term Outcomes of Late-Onset Multiple Sclerosis: A Swedish Nationwide Study
- PMID: 38394472
- PMCID: PMC11033980
- DOI: 10.1212/WNL.0000000000208051
Clinical Characteristics and Long-Term Outcomes of Late-Onset Multiple Sclerosis: A Swedish Nationwide Study
Erratum in
-
Clinical Characteristics and Long-Term Outcomes of Late-Onset Multiple Sclerosis: A Swedish Nationwide Study.Neurology. 2024 Oct 8;103(7):e209461. doi: 10.1212/WNL.0000000000209461. Epub 2024 Sep 11. Neurology. 2024. PMID: 39259918 Free PMC article. No abstract available.
Abstract
Background and objectives: Clinical onset of multiple sclerosis (MS) after the age of 50 years is uncommon and associated with a less favorable natural history. The differences in long-term outcomes in patients with late-onset MS (LOMS, onset 50 years or older) and adult-onset MS (AOMS, onset 18 years or older and younger than 50 years) during the disease-modifying therapy (DMT) era have been less studied. This study aimed to compare patient characteristics, DMT exposure, and disability progression in Swedish patients with LOMS and AOMS over 2 decades (2001-2022).
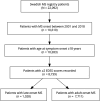
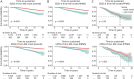
Methods: The nationwide Swedish MS registry was searched for patients with an onset of MS between January 1, 2001, and December 31, 2018, with symptom onset at age 18 years or older and ≥2 recorded Expanded Disability Status Scale (EDSS) scores. Clinical and demographic parameters and exposure to DMT were compared between LOMS and AOMS. Time to disability milestones (EDSS 4 and 6) was assessed using Kaplan-Meier curves and Cox proportional hazards regression models adjusted for sex, disease course, calendar year at onset, and DMT exposure.
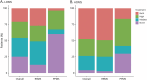
Results: Among 8739 patients with MS who met inclusion criteria, 1,028 (11.8%) were LOMS. Primary progressive MS was more frequently diagnosed in LOMS compared with that in AOMS (25.2% vs 4.5%; p < 0.001). Most of the patients had been prescribed DMT, but more rarely in LOMS compared with AOMS (74.7% vs 95.6%; p < 0.001). Less than half of patients with LOMS had been exposed to a high-efficacy DMT (45.8%) compared with 73.5% of AOMS (p < 0.001). The risk of reaching disability milestones was greater for LOMS compared with that for AOMS (EDSS 4; adjusted hazard ratio [aHR] 2.71; 95% CI 2.22-3.30; p < 0.001, and EDSS 6; aHR 2.67; 95% CI 2.12-3.36; p < 0.001).
Discussion: This study distinguishes LOMS as a particularly vulnerable group and clinically supports close vigilance of these patients. Further studies are needed to assess and clarify the benefit of DMT usage in older adults with MS.
Conflict of interest statement
E.F. Mouresan, E. Mentesidou, and A. Berglund declare no conflict of interest: K.A. McKay has received speaker honoraria from Biogen and Sanofi-Aventis and is funded by the Swedish Research Council for Health, Working Life and Welfare, and StratNeuro. J. Hillert received honoraria for serving on advisory boards for Biogen, Bristol-Myers-Squibb, Janssen, Merck KGaA, Novartis, Sandoz, and Sanofi-Genzyme and speaker's fees from Biogen, Janssen, Novartis, Merck, Teva, Sandoz, and Sanofi-Genzyme. He has served as PI for projects sponsored by, or received unrestricted research support from, Biogen, Bristol-Myers-Squibb, Janssen, Merck KGaA, Novartis, Roche, and Sanofi-Genzyme. His MS research is funded by the Swedish Research Council and the Swedish Brain foundation. E. Iacobaeus has received honoraria for serving on advisory boards for Biogen, Sanofi-Genzyme, and Merck and speaker's fees from Biogen and Sanofi-Genzyme. She has received funding from Region Stockholm Clinical Research Appointment and the Lindholm Fredholms foundation. Go to
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
