Therapeutic efficacy of high-dose chemotherapy with autologous stem-cell transplantation in 44 relapsed or refractory germ-cell tumor patients: A retrospective cohort study
- PMID: 38394499
- PMCID: PMC11309616
- DOI: 10.1097/MD.0000000000037213
Therapeutic efficacy of high-dose chemotherapy with autologous stem-cell transplantation in 44 relapsed or refractory germ-cell tumor patients: A retrospective cohort study
Abstract
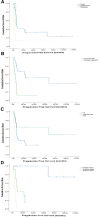
Despite having a higher mortality risk than conventional chemotherapeutics, high-dose chemotherapy (HDCT) has the potential to be curative in relapsed/refractory germ-cell tumors. Therefore, selecting the best patient group for this treatment is critical. This study aimed to determine the factors that affect survival in our relapsed/refractory GCT cohort who received HDCT and autologous stem-cell transplantation. Between September 2010 and 2020, we included in the study 44 relapsed/refractory male patients with GCT treated with HDCT plus autologous stem-cell transplantation. The patients' demographic features, clinical characteristics, and treatment outcomes were evaluated. Statistical analyses were performed to identify risk factors associated with survival. The median age of all cohorts was 28 years. Thirty-six patients had nonseminomatous tumors, and 8 patients had seminomatous tumors. The most common primary tumor sites were the gonads (75%), followed by the mediastinum (15.9%) and the retroperitoneum (9.1%). After HDCT, 11 patients had a complete response, 12 patients had a partial response, and 17 patients had a progressive disease, respectively. About 23 patients (52.3%) experienced at least 1 treatment-related grade 3 to 4 nonhematological toxicity. About 4 patients (10%) died due to HDCT-related toxicity. The total group's median progression-free survival (PFS) was 7 months, and the median overall survival (OS) was 14.9 months. Primary tumor site (hazard ratio [HR]: 1.84; P = .028), type of HDCT regimen (HR: 0.35; P = .010), and best response to HDCT (HR: 11.0; P < .0001) were independent prognostic risk factors for PFS. The only independent prognostic risk factor associated with OS was the best response to HDCT (HR: 6.62; P = .001). The results of the study promise the best response to HDCT as a primary measure for predicting survival in relapsed/refractory GCT. In contrast, primary mediastinal GCT is not a good candidate for HDCT. Furthermore, a carboplatin-etoposide regimen in combination with cyclophosphamide and paclitaxel may improve PFS.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Figures

References
-
- Fitzmaurice C, Allen C, Barber RM, et al. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. - PMC - PubMed
-
- Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–53. - PubMed
-
- Mead G, Stenning S, Cook P, et al. International germ cell consensus classification: A prognostic factor-erased staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. - PubMed
-
- Hanna N, Einhorn LH. Testicular cancer: a reflection on 50 years of discovery. J Clin Oncol. 2014;32:3085–92. - PubMed
-
- Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549–55. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

