A CD38-directed, single-chain T-cell engager targets leukemia stem cells through IFN-γ-induced CD38 expression
- PMID: 38394668
- PMCID: PMC11103097
- DOI: 10.1182/blood.2023021570
A CD38-directed, single-chain T-cell engager targets leukemia stem cells through IFN-γ-induced CD38 expression
Abstract
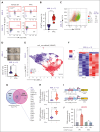
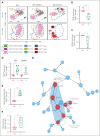
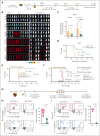
Treatment resistance of leukemia stem cells (LSCs) and suppression of the autologous immune system represent major challenges to achieve a cure in acute myeloid leukemia (AML). Although AML blasts generally retain high levels of surface CD38 (CD38pos), LSCs are frequently enriched in the CD34posCD38neg blast fraction. Here, we report that interferon gamma (IFN-γ) reduces LSCs clonogenic activity and induces CD38 upregulation in both CD38pos and CD38neg LSC-enriched blasts. IFN-γ-induced CD38 upregulation depends on interferon regulatory factor 1 transcriptional activation of the CD38 promoter. To leverage this observation, we created a novel compact, single-chain CD38-CD3 T-cell engager (BN-CD38) designed to promote an effective immunological synapse between CD38pos AML cells and both CD8pos and CD4pos T cells. We demonstrate that BN-CD38 engages autologous CD4pos and CD8pos T cells and CD38pos AML blasts, leading to T-cell activation and expansion and to the elimination of leukemia cells in an autologous setting. Importantly, BN-CD38 engagement induces the release of high levels of IFN-γ, driving the expression of CD38 on CD34posCD38neg LSC-enriched blasts and their subsequent elimination. Critically, although BN-CD38 showed significant in vivo efficacy across multiple disseminated AML cell lines and patient-derived xenograft models, it did not affect normal hematopoietic stem cell clonogenicity and the development of multilineage human immune cells in CD34pos humanized mice. Taken together, this study provides important insights to target and eliminate AML LSCs.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: M. Murtadha, M.P., G.M., J.C.W., and F.P. have pending patents covering certain aspects of this technology in anticipation of commercialization. The remaining authors declare no competing financial interests.
Figures







Comment in
-
IFN-γ and CD38 in AML: a T-cell engagement made in heaven?Blood. 2024 Apr 18;143(16):1556-1557. doi: 10.1182/blood.2023023786. Blood. 2024. PMID: 38635257 No abstract available.
References
-
- Kantarjian H. Acute myeloid leukemia-major progress over four decades and glimpses into the future. Am J Hematol. 2016;91(1):131–145. - PubMed
-
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. - PubMed
-
- Mims AS, Blum W. Progress in the problem of relapsed or refractory acute myeloid leukemia. Curr Opin Hematol. 2019;26(2):88–95. - PubMed
-
- Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117(8):2307–2318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

