Structural Basis for Multivalent MUC16 Recognition and Robust Anti-Pancreatic Cancer Activity of Humanized Antibody AR9.6
- PMID: 38394685
- PMCID: PMC11660185
- DOI: 10.1158/1535-7163.MCT-23-0868
Structural Basis for Multivalent MUC16 Recognition and Robust Anti-Pancreatic Cancer Activity of Humanized Antibody AR9.6
Abstract
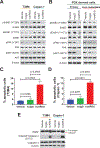
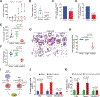
Mucin-16 (MUC16) is a target for antibody-mediated immunotherapy in pancreatic ductal adenocarcinoma (PDAC) among other malignancies. The MUC16-specific monoclonal antibody AR9.6 has shown promise for PDAC immunotherapy and imaging. Here, we report the structural and biological characterization of the humanized AR9.6 antibody (huAR9.6). The structure of huAR9.6 was determined in complex with a MUC16 SEA (Sea urchin sperm, Enterokinase, Agrin) domain. Binding of huAR9.6 to recombinant, shed, and cell-surface MUC16 was characterized, and anti-PDAC activity was evaluated in vitro and in vivo. HuAR9.6 bound a discontinuous, SEA domain epitope with an overall affinity of 88 nmol/L. Binding affinity depended on the specific SEA domain(s) present, and glycosylation modestly enhanced affinity driven by favorable entropy and enthalpy and via distinct transition state thermodynamic pathways. Treatment with huAR9.6 reduced the in vitro growth, migration, invasion, and clonogenicity of MUC16-positive PDAC cells and patient-derived organoids (PDO). HuAR9.6 blocked MUC16-mediated ErbB and AKT activation in PDAC cells, PDOs, and patient-derived xenografts and induced antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. More importantly, huAR9.6 treatment caused substantial PDAC regression in subcutaneous and orthotopic tumor models. The mechanism of action of huAR9.6 may depend on dense avid binding to homologous SEA domains on MUC16. The results of this study validate the translational therapeutic potential of huAR9.6 against MUC16-positive PDACs.
©2024 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest statement
C.L. Brooks received grants from Quest PharmaTech Inc. (AR9.6 patent assignee, US-11773183-B2) during the conduct of the study and grants from Quest PharmaTech Inc. P. Radhakrishnan and M.A. Hollingsworth are inventors on a patent related to huAR9.6 (US-11773183-B2). All other authors declare no competing interests.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. United States; 2023;73:17–48. - PubMed
-
- Marcos-Silva L, Narimatsu Y, Halim A, Campos D, Yang Z, Tarp MA, et al. Characterization of binding epitopes of CA125 monoclonal antibodies. J Proteome Res. United States; 2014;13:3349–59. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

