Cardiac and perivascular myofibroblasts, matrifibrocytes, and immune fibrocytes in hypertension; commonalities and differences with other cardiovascular diseases
- PMID: 38395029
- PMCID: PMC11485269
- DOI: 10.1093/cvr/cvae044
Cardiac and perivascular myofibroblasts, matrifibrocytes, and immune fibrocytes in hypertension; commonalities and differences with other cardiovascular diseases
Abstract
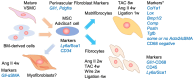
Hypertension is a major cause of cardiovascular diseases such as myocardial infarction and stroke. Cardiovascular fibrosis occurs with hypertension and contributes to vascular resistance, aortic stiffness, and cardiac hypertrophy. However, the molecular mechanisms leading to fibroblast activation in hypertension remain largely unknown. There are two types of fibrosis: replacement fibrosis and reactive fibrosis. Replacement fibrosis occurs in response to the loss of viable tissue to form a scar. Reactive fibrosis occurs in response to an increase in mechanical and neurohormonal stress. Although both types of fibrosis are considered adaptive processes, they become maladaptive when the tissue loss is too large, or the stress persists. Myofibroblasts represent a subpopulation of activated fibroblasts that have gained contractile function to promote wound healing. Therefore, myofibroblasts are a critical cell type that promotes replacement fibrosis. Although myofibroblasts were recognized as the fibroblasts participating in reactive fibrosis, recent experimental evidence indicated there are distinct fibroblast populations in cardiovascular reactive fibrosis. Accordingly, we will discuss the updated definition of fibroblast subpopulations, the regulatory mechanisms, and their potential roles in cardiovascular pathophysiology utilizing new knowledge from various lineage tracing and single-cell RNA sequencing studies. Among the fibroblast subpopulations, we will highlight the novel roles of matrifibrocytes and immune fibrocytes in cardiovascular fibrosis including experimental models of hypertension, pressure overload, myocardial infarction, atherosclerosis, aortic aneurysm, and nephrosclerosis. Exploration into the molecular mechanisms involved in the differentiation and activation of those fibroblast subpopulations may lead to novel treatments for end-organ damage associated with hypertension and other cardiovascular diseases.
Keywords: Fibroblast; Fibrosis; Hypertension; Myocardial Infarction; Pressure overload.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: None declared.
Figures


References
-
- Al-Makki A, DiPette D, Whelton PK, Murad MH, Mustafa RA, Acharya S, Beheiry HM, Champagne B, Connell K, Cooney MT, Ezeigwe N, Gaziano TA, Gidio A, Lopez-Jaramillo P, Khan UI, Kumarapeli V, Moran AE, Silwimba MM, Rayner B, Sukonthasan A, Yu J, Saraffzadegan N, Reddy KS, Khan T. Hypertension pharmacological treatment in adults: a world health organization guideline executive summary. Hypertension 2022;79:293–301. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

