SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase
- PMID: 38395697
- PMCID: PMC11066813
- DOI: 10.1016/j.immuni.2024.01.017
SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase
Abstract
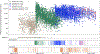
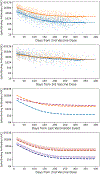
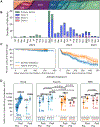
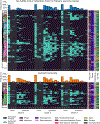
It is thought that mRNA-based vaccine-induced immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) wanes quickly, based mostly on short-term studies. Here, we analyzed the kinetics and durability of the humoral responses to SARS-CoV-2 infection and vaccination using >8,000 longitudinal samples collected over a 3-year period in New York City. Upon primary immunization, participants with pre-existing immunity mounted higher antibody responses faster and achieved higher steady-state antibody titers than naive individuals. Antibody kinetics were characterized by two phases: an initial rapid decay, followed by a stabilization phase with very slow decay. Booster vaccination equalized the differences in antibody concentration between participants with and without hybrid immunity, but the peak antibody titers decreased with each successive antigen exposure. Breakthrough infections increased antibodies to similar titers as an additional vaccine dose in naive individuals. Our study provides strong evidence that SARS-CoV-2 antibody responses are long lasting, with initial waning followed by stabilization.
Keywords: COVID-19 vaccines; SARS-CoV-2 immunity; SARS-CoV-2 variants; antibodies; breakthrough infections; durability; hybrid immunity; longitudinal study; modeling antibody kinetics; reactogenicity.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines that list F.K. as co-inventor. V.S. is also listed on the SARS-CoV-2 serological assays patent. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. F.K. has consulted for CureVac, Merck, and Pfizer, and is currently consulting for 3rd Rock Ventures, GSK, Gritstone, and Avimex, and he is a co-founder and scientific advisory board member of CastleVax. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2.
Figures

References
-
- Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, Sominsky LA, Clark JJ, Adelsberg DC, Bielak DA, et al. (2022). Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 602, 682–688. - PubMed
-
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, et al. (2022). Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 399, 924–944. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

