Overreporting of adherence to hepatitis C direct-acting antiviral therapy and sustained virologic response among people who inject drugs in the HERO study
- PMID: 38395747
- PMCID: PMC10893697
- DOI: 10.1186/s12879-024-09124-3
Overreporting of adherence to hepatitis C direct-acting antiviral therapy and sustained virologic response among people who inject drugs in the HERO study
Abstract
Background: Self-reported adherence to direct-acting antivirals (DAAs) to treat hepatitis C virus (HCV) among persons who inject drugs (PWID) is often an overreport of objectively measured adherence. The association of such overreporting with sustained virologic response (SVR) is understudied. This study among PWID aimed to determine a threshold of overreporting adherence that optimally predicts lower SVR rates, and to explore correlates of the optimal overreporting threshold.
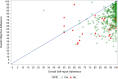
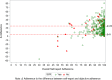
Methods: This study analyzed per-protocol data of participants with adherence data (N = 493) from the HERO (Hepatitis C Real Options) study. Self-reported and objective adherence to a 12-week DAA regimen were measured using visual analogue scales and electronic blister packs, respectively. The difference (Δ) between self-reported and objectively measured adherence was calculated. We used the Youden index based on receiver operating characteristic (ROC) curve analysis to identify an optimal threshold of overreporting for predicting lower SVR rates. Factors associated with the optimal threshold of overreporting were identified by comparing baseline characteristics between participants at/above versus those below the threshold.
Results: The self-reported, objective, and Δ adherence averages were 95.1% (SD = 8.9), 75.9% (SD = 16.3), and 19.2% (SD = 15.2), respectively. The ≥ 25% overreporting threshold was determined to be optimal. The SVR rate was lower for ≥ 25% vs. < 25% overreporting (86.7% vs. 95.8%, p <.001). The factors associated with ≥ 25% Δ adherence were unemployment; higher number of days and times/day of injecting drugs; higher proportion of positive urine drug screening for amphetamine, methamphetamine, and oxycodone, and negative urine screening for THC (tetrahydrocannabinol)/cannabis.
Conclusions: Self-reported DAA adherence was significantly greater than objectively measured adherence among PWID by 19.2%. Having ≥ 25% overreported adherence was associated with optimal prediction of lower SVR rates. PWID with risk factors for high overreporting may need to be more intensively managed to promote actual adherence.
Keywords: Adherence; Electronic blister pack; HCV DAA; Objective measure; Overreporting; Persons who inject drugs; SVR; Self-report; Visual analog scale.
© 2024. The Author(s).
Conflict of interest statement
JF has received research grant support from Gilead Sciences. AYK has served on advisory boards for Biomarin. AHL has served on advisory boards for Gilead Sciences and Merck Pharmaceuticals and received research funding from Gilead Sciences. SHM has received speaker fees from Gilead Sciences. All other authors declare no competing interests.
Figures
Similar articles
-
Optimal hepatitis C treatment adherence patterns and sustained virologic response among people who inject drugs: The HERO study.J Hepatol. 2024 May;80(5):702-713. doi: 10.1016/j.jhep.2023.12.020. Epub 2024 Jan 17. J Hepatol. 2024. PMID: 38242324 Clinical Trial.
-
Self-reported and measured adherence to hepatitis C direct-acting antiviral therapy and sustained virologic response among people who inject drugs: The HERO study.Int J Drug Policy. 2024 Jan;123:104288. doi: 10.1016/j.drugpo.2023.104288. Epub 2023 Dec 15. Int J Drug Policy. 2024. PMID: 38103458 Clinical Trial.
-
Intensive Models of Hepatitis C Care for People Who Inject Drugs Receiving Opioid Agonist Therapy: A Randomized Controlled Trial.Ann Intern Med. 2019 May 7;170(9):594-603. doi: 10.7326/M18-1715. Epub 2019 Apr 9. Ann Intern Med. 2019. PMID: 30959528 Free PMC article. Clinical Trial.
-
Efficacy of Direct-acting Antivirals for Chronic Hepatitis C Virus Infection in People Who Inject Drugs or Receive Opioid Substitution Therapy: A Systematic Review and Meta-analysis.Clin Infect Dis. 2020 May 23;70(11):2355-2365. doi: 10.1093/cid/ciz696. Clin Infect Dis. 2020. PMID: 31513710
-
Staying hepatitis C negative: A systematic review and meta-analysis of cure and reinfection in people who inject drugs.Liver Int. 2019 Dec;39(12):2244-2260. doi: 10.1111/liv.14152. Epub 2019 Jun 10. Liver Int. 2019. PMID: 31125496
Cited by
-
Prevalence of Hepatitis C in the Emilia-Romagna Region of Italy: Population-Wide Screening.Viruses. 2025 Jun 12;17(6):843. doi: 10.3390/v17060843. Viruses. 2025. PMID: 40573434 Free PMC article.
-
Peer-Assisted Telemedicine for Hepatitis C in People Who Use Drugs: A Randomized Controlled Trial.Clin Infect Dis. 2025 Mar 17;80(3):501-508. doi: 10.1093/cid/ciae520. Clin Infect Dis. 2025. PMID: 39602441 Clinical Trial.
References
-
- Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report– United States, 2020 2022, September [cited 2023 6/27/2023]. Available from: https://www.cdc.gov/hepatitis/statistics/2020surveillance/hepatitis-c.htm.
-
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Global Health. 2017;5(12):E1192–E207. doi: 10.1016/S2214-109X(17)30375-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
- HPC-1503-28122/PCORI/Patient-Centered Outcomes Research Institute/United States
LinkOut - more resources
Full Text Sources
Medical