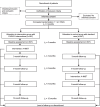
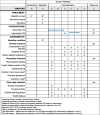
PAIN2.0: study protocol for a multicentre randomised controlled trial to evaluate the efficacy of a 10-week outpatient interdisciplinary multimodal pain therapy to manage recurrent pain for patients with risk factors of developing chronic pain in Germany
- PMID: 38395869
- PMCID: PMC10893721
- DOI: 10.1186/s13063-024-07975-4
PAIN2.0: study protocol for a multicentre randomised controlled trial to evaluate the efficacy of a 10-week outpatient interdisciplinary multimodal pain therapy to manage recurrent pain for patients with risk factors of developing chronic pain in Germany
Abstract
Background: Up to 27% of the German population suffers from recurrent or persistent pain (lasting more than three months). Therefore, prevention of chronic pain is one major object of pain management interventions. The aim of this nationwide, multicentre, randomised controlled trial is to evaluate the efficacy of a 10-week ambulatory (outpatient) interdisciplinary multimodal pain therapy (A-IMPT) for patients with recurrent pain and at risk of developing chronic pain. This project was initiated by the German Pain Society (Deutsche Schmerzgesellschaft e.V.) and the public health insurance provider BARMER. It is currently funded by the German Innovation Fund (01NVF20023). The study PAIN2.0 focuses on reducing pain intensity and pain-related disability and investigates whether this intervention can improve physical activity, psychological well-being, and health literacy.
Methods: PAIN2.0 is designed as a multicentre 1:1 randomised controlled trial with two parallel groups (randomisation at the patient level, planned N = 1094, duration of study participation 12 months, implemented by 22 health care facilities nationwide). After 6 months, patients within the control group also receive the intervention. The primary outcomes are pain intensity and pain-related impairment, measured as Characteristic Pain Intensity (PI) and Disability Score (DS) (Von Korff), as well as patient-related satisfaction with the intervention. Secondary outcomes are the number of sick leave days, sickness allowance, treatment costs, psychological distress, health-related quality of life, and catastrophizing. The effects of the intervention will be analysed by a parallel-group comparison between the intervention and control groups. In addition, the long-term effects within the intervention group will be observed and a pre-post comparison of the control group before and after the intervention will be performed.
Discussion: Recurrent or persistent pain is common in the German population and causes high costs for patients and society. The A-IMPT aims to improve pain and pain-related impairments in pain patients at risk of chronification, thereby reducing the risk of developing chronic pain with its high socioeconomic burden. This new therapy could easily be integrated into existing therapy programs if positively evaluated.
Trial registration: The trial PAIN2.0 has been registered in the German Clinical Trials Register (DRKS) since 21/11/2022 with the ID DRKS00030773 .
Keywords: Complex intervention; Health-related quality of life; Mixed models for repeated measures; Multimodal interdisciplinary pain management; Outpatient group therapy; Pain and risk factors; Public health; Randomised controlled trial; Recurrent pain; Secondary prevention.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- German Clinical Trials Register. https://drks.de/search/de/trial/DRKS00030773. Accessed 13 Sept 2023.
-
- International Association for the Study of Pain. https://www.iasp-pain.org/advocacy/global-year/prevention-of-pain/#:~:te.... Accessed 20 Sept 2023.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous