Protocol for the ONLOOP trial: pragmatic randomized trial evaluating a province-wide system of personalized reminders for evidence-based surveillance tests in adult survivors of childhood cancer in Ontario
- PMID: 38395903
- PMCID: PMC10885391
- DOI: 10.1186/s13012-024-01347-x
Protocol for the ONLOOP trial: pragmatic randomized trial evaluating a province-wide system of personalized reminders for evidence-based surveillance tests in adult survivors of childhood cancer in Ontario
Abstract
Background: Childhood cancer treatment while often curative, leads to elevated risks of morbidity and mortality. Survivors require lifelong periodic surveillance for late effects of treatment, yet adherence to guideline-recommended tests is suboptimal. We created ONLOOP to provide adult survivors of childhood cancer with detailed health information, including summaries of their childhood cancer treatment and recommended surveillance tests for early detection of cardiomyopathy, breast cancer, and/or colorectal cancer, with personalized reminders over time.
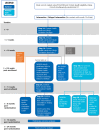
Methods: This is an individually randomized, registry-based pragmatic trial with an embedded process and economic evaluation to understand ONLOOP's impact and whether it can be readily implemented at scale. All adult survivors of childhood cancer in Ontario overdue for guideline-recommended tests will be randomly assigned to one of two arms: (1) intervention or (2) delayed intervention. A letter of information and invitation will detail the ONLOOP program. Those who sign up will receive a personalized toolkit and a screening reminder 6 months later. With the participants' consent, ONLOOP will also send their primary care clinician a letter detailing the recommended tests and a reminder 6 months later. The primary outcome will be the proportion of survivors who complete one or more of the guideline-recommended cardiac, breast, or colon surveillance tests during the 12 months after randomization. Data will be obtained from administrative databases. The intent-to-treat principle will be followed. Based on our analyses of administrative data, we anticipate allocating at least 862 individuals to each trial arm, providing 90% power to detect an absolute increase of 6% in targeted surveillance tests completed. We will interview childhood cancer survivors and family physicians in an embedded process evaluation to examine why and how ONLOOP achieved success or failed. A cost-effectiveness evaluation will be performed.
Discussion: The results of this study will determine if ONLOOP is effective at helping adult survivors of childhood cancer complete their recommended surveillance tests. This study will also inform ongoing provincial programs for this high-risk population.
Trial registration: ClinicalTrials.gov NCT05832138.
© 2024. The Author(s).
Conflict of interest statement
Jeremy Grimshaw and Noah Ivers are members of the Implementation Science editorial board. All other authors declare that they have no competing interests.
Figures
Similar articles
-
Longitudinal adherence to surveillance for late effects of cancer treatment: a population-based study of adult survivors of childhood cancer.CMAJ. 2024 Mar 10;196(9):E282-E294. doi: 10.1503/cmaj.231358. CMAJ. 2024. PMID: 38467416 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: a systematic review.J Cancer Surviv. 2019 Oct;13(5):713-729. doi: 10.1007/s11764-019-00790-w. Epub 2019 Jul 24. J Cancer Surviv. 2019. PMID: 31338733
-
Developing a Provincial Surveillance and Support System for Childhood Cancer Survivors: Multiphase User-Centered Design Study.JMIR Hum Factors. 2022 Sep 13;9(3):e37606. doi: 10.2196/37606. JMIR Hum Factors. 2022. PMID: 36099013 Free PMC article.
-
Coronary artery disease surveillance among childhood, adolescent and young adult cancer survivors: A systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group.Eur J Cancer. 2021 Oct;156:127-137. doi: 10.1016/j.ejca.2021.06.021. Epub 2021 Aug 24. Eur J Cancer. 2021. PMID: 34450551
Cited by
-
Design thinking in cancer care: A systematic literature review.Digit Health. 2025 May 21;11:20552076241313279. doi: 10.1177/20552076241313279. eCollection 2025 Jan-Dec. Digit Health. 2025. PMID: 40416070 Free PMC article. Review.
References
-
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. - DOI - PubMed
-
- Group. CsO . long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. 2018.
-
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. - DOI - PubMed
-
- Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 3.0. 2008. http://www.survivor-shipguidelines.org/.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical