Lithium response in bipolar disorder is associated with focal adhesion and PI3K-Akt networks: a multi-omics replication study
- PMID: 38395906
- PMCID: PMC10891068
- DOI: 10.1038/s41398-024-02811-4
Lithium response in bipolar disorder is associated with focal adhesion and PI3K-Akt networks: a multi-omics replication study
Abstract
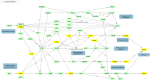
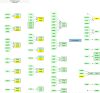
Lithium is the gold standard treatment for bipolar disorder (BD). However, its mechanism of action is incompletely understood, and prediction of treatment outcomes is limited. In our previous multi-omics study of the Pharmacogenomics of Bipolar Disorder (PGBD) sample combining transcriptomic and genomic data, we found that focal adhesion, the extracellular matrix (ECM), and PI3K-Akt signaling networks were associated with response to lithium. In this study, we replicated the results of our previous study using network propagation methods in a genome-wide association study of an independent sample of 2039 patients from the International Consortium on Lithium Genetics (ConLiGen) study. We identified functional enrichment in focal adhesion and PI3K-Akt pathways, but we did not find an association with the ECM pathway. Our results suggest that deficits in the neuronal growth cone and PI3K-Akt signaling, but not in ECM proteins, may influence response to lithium in BD.
© 2024. The Author(s).
Conflict of interest statement
MAd has received a grant from Servier, speaker’s fees from Servier, Lundbeck, Aristo, Parexel, Gilead, ViiV, Deutsche Bank, MSD, and MyTomorrows, plus a non-financial support from Lundbeck. KA has received speaker’s fees from Taisho Toyama Pharmaceutical. MAl is funded by a grant of the Canadian Institutes of Health Research. MB has received speaker’s fees from AstraZeneca, Pfizer, Lilly, Lundbeck, GlaxoSmithKline, Servier, and Ferrer Internacional. BÉ received non-financial support from Labex Biopsy and Fondation Fondamental. RH received grants and speaker honoraria from Dainippon Sumitomo Pharma and Novartis plus speaker honoraria from Eli Lilly Japan, GlaxoSmithKline, Hisamitsu Pharmaceutical, Janssen Pharmaceutical, Nippon Zoki Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Pfizer, and the Yoshitomiyakuhin Corporation. TK received a grant from Takeda Pharmaceutical and fees from Kyowa Hakko Kirin, Eli Lilly Japan, Otsuka Pharmaceutical, GlaxoSmithKline, Taisho Toyama Pharmaceutical, Dainippon Sumitomo Pharma, Meiji Seika Pharma, Pfizer Japan, Mochida Pharmaceutical, Shionogi & Co, Janssen Pharmaceutical, Yoshitomiyakuhin Corporation, Agilent Technologies, Astellas Pharma, and Wako Pure Chemical Industries. IK received grants and fees from Dainippon Sumitomo Pharma, Eisai, Eli Lilly, GlaxoSmithKline, Kyowa Hakko Kirin, Meiji Seika Pharma, MSD, Novartis, Otsuka, Ono Pharmaceutical, Pfizer, Tanabe Mitsubishi Pharma, Takeda Pharmaceutical, Shionogi, and Yoshitomi Pharmaceutical; he received grants from AbbVie GK, Asahi Kasei Pharma, Boehringer Ingelheim, Chugai Pharmaceutical, and Daiichi Sankyo and fees from Astellas Pharma and Janssen Pharmaceutical. MJM served as unpaid consultant for Pathway Genomic (San Diego, USA). SLM received a grant and fees from Naurex and Shire, further grants from Alkermes, Cephalon, Forest, Marriott Foundation, Orexigen Therapeutics, and Takeda Pharmaceutical, he further has served on the advisory boards for Bracket, Hoffmann-La Roche, MedAvante, Sunovion and received fees from Novo Nordisk. RHP received personal fees from RID Ventures, Genomind LLC, Healthrageous, Pfizer, Perfect Health, Proteus, and Psybrain. PRS received a grant from NHMRC. TGS received a grant and fees from Roche Pharmaceuticals. TSt received personal fees from Servier, Lundbeck, and Bristol-Myers Squibb. MMan has received grants from Lundbeck and Angelini. EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, AbbVie, Adamed, Angelini, Biogen, Biohaven, Boehringer-Ingelheim, Celon Pharma, Compass, Dainippon Sumitomo Pharma, Ethypharm, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, HMNC, Idorsia, Janssen, Lundbeck, Medincell, Merck, Novartis, Orion Corporation, Organon, Otsuka, Roche, Rovi, Sage, Sanofi-Aventis, Sunovion, Takeda, and Viatris, outside the submitted work. SRC has participated in advisory and educational boards and received speaker’s fees from Janssen-Cilag, Lundbeck, Otsuka, and Servier; research funding from Janssen-Cilag, Lundbeck, Otsuka, and Gilead; and data sharing from Viatris Australia. ML has received lecture honoraria from Lundbeck pharmaceuticals. All above listed interests are outside of the submitted work. All other authors declare no competing interests.
Figures

Update of
-
Lithium Response in Bipolar Disorder is Associated with Focal Adhesion and PI3K-Akt Networks: A Multi-omics Replication Study.Res Sq [Preprint]. 2023 Oct 3:rs.3.rs-3258813. doi: 10.21203/rs.3.rs-3258813/v1. Res Sq. 2023. Update in: Transl Psychiatry. 2024 Feb 23;14(1):109. doi: 10.1038/s41398-024-02811-4. PMID: 37886563 Free PMC article. Updated. Preprint.
References
-
- Sajatovic M. Bipolar disorder: disease burden. Am J Manag Care. 2005;11:S80–4. - PubMed
MeSH terms
Substances
Grants and funding
- R01 MH059556/MH/NIMH NIH HHS/United States
- R01 MH059535/MH/NIMH NIH HHS/United States
- R01 MH059567/MH/NIMH NIH HHS/United States
- R01 MH059545/MH/NIMH NIH HHS/United States
- P01 CA089392/CA/NCI NIH HHS/United States
- Z01 MH002810/ImNIH/Intramural NIH HHS/United States
- I01 BX003431/BX/BLRD VA/United States
- R01 MH059548/MH/NIMH NIH HHS/United States
- R01 MH059533/MH/NIMH NIH HHS/United States
- U01 MH092758/MH/NIMH NIH HHS/United States
- K02 DA021237/DA/NIDA NIH HHS/United States
- R01 MH059553/MH/NIMH NIH HHS/United States
- R01 MH060068/MH/NIMH NIH HHS/United States
- MR/L006642/1/MRC_/Medical Research Council/United Kingdom
- R01 MH059534/MH/NIMH NIH HHS/United States

