Influence of microbiota-associated metabolic reprogramming on clinical outcome in patients with melanoma from the randomized adjuvant dendritic cell-based MIND-DC trial
- PMID: 38395948
- PMCID: PMC10891084
- DOI: 10.1038/s41467-024-45357-1
Influence of microbiota-associated metabolic reprogramming on clinical outcome in patients with melanoma from the randomized adjuvant dendritic cell-based MIND-DC trial
Abstract
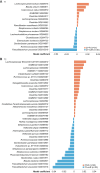
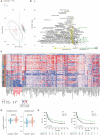
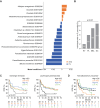
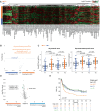
Tumor immunosurveillance plays a major role in melanoma, prompting the development of immunotherapy strategies. The gut microbiota composition, influencing peripheral and tumoral immune tonus, earned its credentials among predictors of survival in melanoma. The MIND-DC phase III trial (NCT02993315) randomized (2:1 ratio) 148 patients with stage IIIB/C melanoma to adjuvant treatment with autologous natural dendritic cell (nDC) or placebo (PL). Overall, 144 patients collected serum and stool samples before and after 2 bimonthly injections to perform metabolomics (MB) and metagenomics (MG) as prespecified exploratory analysis. Clinical outcomes are reported separately. Here we show that different microbes were associated with prognosis, with the health-related Faecalibacterium prausnitzii standing out as the main beneficial taxon for no recurrence at 2 years (p = 0.008 at baseline, nDC arm). Therapy coincided with major MB perturbations (acylcarnitines, carboxylic and fatty acids). Despite randomization, nDC arm exhibited MG and MB bias at baseline: relative under-representation of F. prausnitzii, and perturbations of primary biliary acids (BA). F. prausnitzii anticorrelated with BA, medium- and long-chain acylcarnitines. Combined, these MG and MB biomarkers markedly determined prognosis. Altogether, the host-microbial interaction may play a role in localized melanoma. We value systematic MG and MB profiling in randomized trials to avoid baseline differences attributed to host-microbe interactions.
© 2024. The Author(s).
Conflict of interest statement
L.Zi. founded EverImmune and is the SAB president of EverImmune. L.ZI. had grant support from Daichi Sankyo, Kaleido, 9 meters and Pileje. A.M.M.E.: consulting fees from Acetra, Agenus, BioInvent, Brenus, CatalYm, Epics, Ellipses, Galecto, GenOway, IO Biotech, IQVIA, ISA Pharmaceuticals, Merck&Co, MSD, Pierre Fabre, Sairopa, Scorpion, Sellas, SkylineDX, TigeTx, Trained Immunity TX. A.M.M.E.: participation on a Data Safety Monitoring Board: Boehringer Ingelheim, BioNTech, and Pfizer. A.M.M.E.: lectures for BMS, MSD. A.M.M.E.: stock or stock options for IO Biotech, SkylineDx and Sairopa. G.K. has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys, and Vascage. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific co-founder of EverImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. G.K. is in the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. G.K. is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. G.K.’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. B.R. is co-founder of Science Curebiota. L.D. is a SAB member of EverImmune. K.F.B.: consulting fees (institutional) from MSD and Pierre Fabre. The remaining authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

