Optimizing protein delivery rate from silk fibroin hydrogel using silk fibroin-mimetic peptides conjugation
- PMID: 38395958
- PMCID: PMC10891107
- DOI: 10.1038/s41598-024-53689-7
Optimizing protein delivery rate from silk fibroin hydrogel using silk fibroin-mimetic peptides conjugation
Abstract
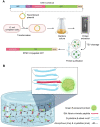
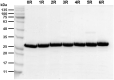
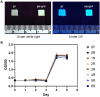
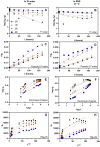
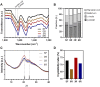
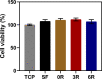
Controlled release of proteins, such as growth factors, from biocompatible silk fibroin (SF) hydrogel is valuable for its use in tissue engineering, drug delivery, and other biological systems. To achieve this, we introduced silk fibroin-mimetic peptides (SFMPs) with the repeating unit (GAGAGS)n. Using green fluorescent protein (GFP) as a model protein, our results showed that SFMPs did not affect the GFP function when conjugated to it. The SFMP-GFP conjugates incorporated into SF hydrogel did not change the gelation time and allowed for controlled release of the GFP. By varying the length of SFMPs, we were able to modulate the release rate, with longer SFMPs resulting in a slower release, both in water at room temperature and PBS at 37 °C. Furthermore, the SF hydrogel with the SFMPs showed greater strength and stiffness. The increased β-sheet fraction of the SF hydrogel, as revealed by FTIR analysis, explained the gel properties and protein release behavior. Our results suggest that the SFMPs effectively control protein release from SF hydrogel, with the potential to enhance its mechanical stability. The ability to modulate release rates by varying the SFMP length will benefit personalized and controlled protein delivery in various systems.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Preparation and characterization of silk fibroin hydrogel as injectable implants for sustained release of Risperidone.Drug Dev Ind Pharm. 2018 Feb;44(2):199-205. doi: 10.1080/03639045.2017.1386195. Epub 2017 Oct 23. Drug Dev Ind Pharm. 2018. PMID: 28956466
-
Synthesis of pH and Glucose Responsive Silk Fibroin Hydrogels.Int J Mol Sci. 2021 Jul 1;22(13):7107. doi: 10.3390/ijms22137107. Int J Mol Sci. 2021. PMID: 34281160 Free PMC article.
-
Novel silk fibroin nanoparticles incorporated silk fibroin hydrogel for inhibition of cancer stem cells and tumor growth.Int J Nanomedicine. 2018 Sep 17;13:5405-5418. doi: 10.2147/IJN.S166104. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30271137 Free PMC article.
-
Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels.Acta Biomater. 2021 Apr 15;125:57-71. doi: 10.1016/j.actbio.2021.02.018. Epub 2021 Feb 16. Acta Biomater. 2021. PMID: 33601067 Review.
-
Silk protein-based hydrogels: Promising advanced materials for biomedical applications.Acta Biomater. 2016 Feb;31:17-32. doi: 10.1016/j.actbio.2015.11.034. Epub 2015 Nov 18. Acta Biomater. 2016. PMID: 26602821 Review.
Cited by
-
Biomimetic peptide conjugates as emerging strategies for controlled release from protein-based materials.Drug Deliv. 2025 Dec;32(1):2449703. doi: 10.1080/10717544.2025.2449703. Epub 2025 Jan 9. Drug Deliv. 2025. PMID: 39782014 Free PMC article. Review.
-
Protein-Based Silver Nanoparticles: Synthesis, Characterization, Administration, and Nanomedicine Applications.Int J Biomater. 2025 May 26;2025:5533798. doi: 10.1155/ijbm/5533798. eCollection 2025. Int J Biomater. 2025. PMID: 40458607 Free PMC article. Review.
References
-
- Manissorn J, Wattanachai P, Tonsomboon K, Bumroongsakulsawat P, Damrongsakkul S, Thongnuek P. Osteogenic enhancement of silk fibroin-based bone scaffolds by forming hybrid composites with bioactive glass through GPTMS during sol-gel process. Mater. Today Commun. 2021;26:101730. doi: 10.1016/j.mtcomm.2020.101730. - DOI
MeSH terms
Substances
Grants and funding
- RAF_2562_012/Research Assistantship Funding from Faculty of Science, Chulalongkorn University
- B05F640156/Program Management Unit for Human Resources & Institutional Development, Research, and Innovation
- 2020R1I1A1A01073559/Basic Science Research Program through the National Research Foundation of Korea (NRF) by the Ministry of Education
- 2021H1D3A2A02045561/Brain Pool program of the National Research Foundation (NRF) by the Korean government (MOE and MSIT)
- RS-2023-00208587/Brain Pool program of the National Research Foundation (NRF) by the Korean government (MOE and MSIT)
- CU_FRB65_hea (74)_168_21_34/Thailand Science research and Innovation Fund Chulalongkorn University
- RES_66_111_2100_011/The Asahi Glass Foundation through CU-AGF grant 2022
- 08/2559/Institute for the Promotion of Teaching Science and Technology (IPST) under the Research Fund for DPST Graduate with First Placement
- CE66-042_2300_009/Center of Excellence in Molecular Crop, Chulalongkorn University
LinkOut - more resources
Full Text Sources

