Stimulating VAPB-PTPIP51 ER-mitochondria tethering corrects FTD/ALS mutant TDP43 linked Ca2+ and synaptic defects
- PMID: 38395965
- PMCID: PMC10885568
- DOI: 10.1186/s40478-024-01742-x
Stimulating VAPB-PTPIP51 ER-mitochondria tethering corrects FTD/ALS mutant TDP43 linked Ca2+ and synaptic defects
Abstract
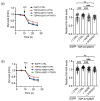
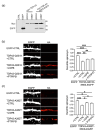
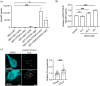
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are clinically linked major neurodegenerative diseases. Notably, TAR DNA-binding protein-43 (TDP43) accumulations are hallmark pathologies of FTD/ALS and mutations in the gene encoding TDP43 cause familial FTD/ALS. There are no cures for FTD/ALS. FTD/ALS display damage to a broad range of physiological functions, many of which are regulated by signaling between the endoplasmic reticulum (ER) and mitochondria. This signaling is mediated by the VAPB-PTPIP51 tethering proteins that serve to recruit regions of ER to the mitochondrial surface so as to facilitate inter-organelle communications. Several studies have now shown that disrupted ER-mitochondria signaling including breaking of the VAPB-PTPIP51 tethers are features of FTD/ALS and that for TDP43 and other familial genetic FTD/ALS insults, this involves activation of glycogen kinase-3β (GSK3β). Such findings have prompted suggestions that correcting damage to ER-mitochondria signaling and the VAPB-PTPIP51 interaction may be broadly therapeutic. Here we provide evidence to support this notion. We show that overexpression of VAPB or PTPIP51 to enhance ER-mitochondria signaling corrects mutant TDP43 induced damage to inositol 1,4,5-trisphosphate (IP3) receptor delivery of Ca2+ to mitochondria which is a primary function of the VAPB-PTPIP51 tethers, and to synaptic function. Moreover, we show that ursodeoxycholic acid (UDCA), an FDA approved drug linked to FTD/ALS and other neurodegenerative diseases therapy and whose precise therapeutic target is unclear, corrects TDP43 linked damage to the VAPB-PTPIP51 interaction. We also show that this effect involves inhibition of TDP43 mediated activation of GSK3β. Thus, correcting damage to the VAPB-PTPIP51 tethers may have therapeutic value for FTD/ALS and other age-related neurodegenerative diseases.
Keywords: Alzheimer’s disease; Amyotrophic lateral sclerosis; Frontotemporal dementia; Neurodegenerative diseases; Parkinson’s disease; TDP43.
© 2024. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures




References
-
- Chand KK, Lee KM, Lee JD, Qiu H, Willis EF, Lavidis NA, Hilliard MA, Noakes PG. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43(Q331K) transgenic mouse model of amyotrophic lateral sclerosis. Faseb J. 2018;32:2676–2689. doi: 10.1096/fj.201700835R. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

