Adjuvant dendritic cell therapy in stage IIIB/C melanoma: the MIND-DC randomized phase III trial
- PMID: 38395969
- PMCID: PMC10891118
- DOI: 10.1038/s41467-024-45358-0
Adjuvant dendritic cell therapy in stage IIIB/C melanoma: the MIND-DC randomized phase III trial
Abstract
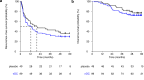
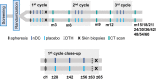
Autologous natural dendritic cells (nDCs) treatment can induce tumor-specific immune responses and clinical responses in cancer patients. In this phase III clinical trial (NCT02993315), 148 patients with resected stage IIIB/C melanoma were randomized to adjuvant treatment with nDCs (n = 99) or placebo (n = 49). Active treatment consisted of intranodally injected autologous CD1c+ conventional and plasmacytoid DCs loaded with tumor antigens. The primary endpoint was the 2-year recurrence-free survival (RFS) rate, whereas the secondary endpoints included median RFS, 2-year and median overall survival, adverse event profile, and immunological response The 2-year RFS rate was 36.8% in the nDC treatment group and 46.9% in the control group (p = 0.31). Median RFS was 12.7 months vs 19.9 months, respectively (hazard ratio 1.25; 90% CI: 0.88-1.79; p = 0.29). Median overall survival was not reached in both treatment groups (hazard ratio 1.32; 90% CI: 0.73-2.38; p = 0.44). Grade 3-4 study-related adverse events occurred in 5% and 6% of patients. Functional antigen-specific T cell responses could be detected in 67.1% of patients tested in the nDC treatment group vs 3.8% of patients tested in the control group (p < 0.001). In conclusion, while adjuvant nDC treatment in stage IIIB/C melanoma patients generated specific immune responses and was well tolerated, no benefit in RFS was observed.
© 2024. The Author(s).
Conflict of interest statement
K.F.B.: consultancy fees (all paid to institute) from MSD and Pierre Fabre. A.A.v.d.V.: consultancy fees (all paid to institute) from BMS, MSD, Pierre Fabre, Merck, Pfizer, Novartis, Sanofi, Ipsen, Eisai, Roche. CA/MBr/KP/AD: employees of Miltenyi Biotec. J.W.B.d.G.: received personal fees outside the submitted work from Bristol-Myers Squibb, Roche, Pierre Fabre, Servier, MSD, and Novartis. J.B.A.G.H.: advisory relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Celsius Therapeutics, GSK, Immunocore, Ipsen, MSD, Merck Serono, Novartis, Neon Therapeutics, Pfizer, Roche/Genentech, Sanofi, and Seattle Genetics and has received research grants not related to this paper from Novartis, BMS, MSD, and Neon Therapeutics. W.R.G.: speaker fees from MSD; advisory role (institutional) for Bristol-Myers Squibb and Bayer; research grants (institutional) from Astellas, Bayer, Janssen-Cilag, MSD. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

