Effect of antipsychotic drugs on group II metabotropic glutamate receptor expression and epigenetic control in postmortem brains of schizophrenia subjects
- PMID: 38396013
- PMCID: PMC10891050
- DOI: 10.1038/s41398-024-02832-z
Effect of antipsychotic drugs on group II metabotropic glutamate receptor expression and epigenetic control in postmortem brains of schizophrenia subjects
Abstract
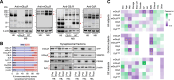
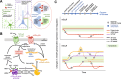
Antipsychotic-induced low availability of group II metabotropic glutamate receptors (including mGlu2R and mGlu3R) in brains of schizophrenia patients may explain the limited efficacy of mGlu2/3R ligands in clinical trials. Studies evaluating mGlu2/3R levels in well-designed, large postmortem brain cohorts are needed to address this issue. Postmortem samples from the dorsolateral prefrontal cortex of 96 schizophrenia subjects and matched controls were collected. Toxicological analyses identified cases who were (AP+) or were not (AP-) receiving antipsychotic treatment near the time of death. Protein and mRNA levels of mGlu2R and mGlu3R, as well as GRM2 and GRM3 promoter-attached histone posttranslational modifications, were quantified. Experimental animal models were used to compare with data obtained in human tissues. Compared to matched controls, schizophrenia cortical samples had lower mGlu2R protein amounts, regardless of antipsychotic medication. Downregulation of mGlu3R was observed in AP- schizophrenia subjects only. Greater predicted occupancy values of dopamine D2 and serotonin 5HT2A receptors correlated with higher density of mGlu3R, but not mGlu2R. Clozapine treatment and maternal immune activation in rodents mimicked the mGlu2R, but not mGlu3R regulation observed in schizophrenia brains. mGlu2R and mGlu3R mRNA levels, and the epigenetic control mechanisms did not parallel the alterations at the protein level, and in some groups correlated inversely. Insufficient cortical availability of mGlu2R and mGlu3R may be associated with schizophrenia. Antipsychotic treatment may normalize mGlu3R, but not mGlu2R protein levels. A model in which epigenetic feedback mechanisms controlling mGlu3R expression are activated to counterbalance mGluR loss of function is described.
© 2024. The Author(s).
Conflict of interest statement
WGH is a consultant with AbbVie, Newron, and Translational Life Sciences. Other authors do not have any financial disclosures to report.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- PID2022-137848OB-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- RTI2018-094414-A-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- RYC-2016-19282/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- BIO22/ALZ/002/Eusko Jaurlaritza (Basque Government)
- IT1512/22/Eusko Jaurlaritza (Basque Government)
LinkOut - more resources
Full Text Sources
Medical

