Gonococcal OMV-delivered PorB induces epithelial cell mitophagy
- PMID: 38396029
- PMCID: PMC10891091
- DOI: 10.1038/s41467-024-45961-1
Gonococcal OMV-delivered PorB induces epithelial cell mitophagy
Abstract
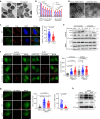
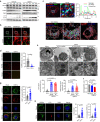
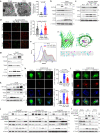
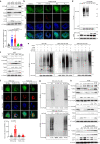
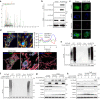
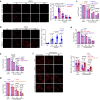
The bacterial pathogen Neisseria gonorrhoeae is able to invade epithelial cells and survive intracellularly. During this process, it secretes outer membrane vesicles (OMVs), however, the mechanistic details for interactions between gonococcal OMVs and epithelial cells and their impact on intracellular survival are currently not established. Here, we show that gonococcal OMVs induce epithelial cell mitophagy to reduce mitochondrial secretion of reactive oxygen species (ROS) and enhance intracellular survival. We demonstrate that OMVs deliver PorB to mitochondria to dissipate the mitochondrial membrane potential, resulting in mitophagy induction through a conventional PINK1 and OPTN/NDP52 mechanism. Furthermore, PorB directly recruits the E3 ubiquitin ligase RNF213, which decorates PorB lysine residue 171 with K63-linked polyubiquitin to induce mitophagy in a p62-dependent manner. These results demonstrate a mechanism in which polyubiquitination of a bacterial virulence factor that targets mitochondria directs mitophagy processes to this organelle to prevent its secretion of deleterious ROS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

