Structural control of corneal transparency, refractive power and dynamics
- PMID: 38396030
- PMCID: PMC11885422
- DOI: 10.1038/s41433-024-02969-7
Structural control of corneal transparency, refractive power and dynamics
Abstract
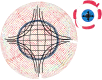
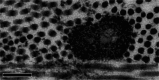
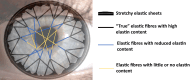
The cornea needs to be transparent to visible light and precisely curved to provide the correct refractive power. Both properties are governed by its structure. Corneal transparency arises from constructive interference of visible light due to the relatively ordered arrangement of collagen fibrils in the corneal stroma. The arrangement is controlled by the negatively charged proteoglycans surrounding the fibrils. Small changes in fibril organisation can be tolerated but larger changes cause light scattering. Corneal keratocytes do not scatter light because their refractive index matches that of the surrounding matrix. When activated, however, they become fibroblasts that have a lower refractive index. Modelling shows that this change in refractive index significantly increases light scatter. At the microscopic level, the corneal stroma has a lamellar structure, the parallel collagen fibrils within each lamella making a large angle with those of adjacent lamellae. X-ray scattering has shown that the lamellae have preferred orientations in the human cornea: inferior-superior and nasal-temporal in the central cornea and circumferential at the limbus. The directions at the centre of the cornea may help withstand the pull of the extraocular muscles whereas the pseudo-circular arrangement at the limbus supports the change in curvature between the cornea and sclera. Elastic fibres are also present; in the limbus they contain fibrillin microfibrils surrounding an elastin core, whereas at the centre of the cornea, they exist as thin bundles of fibrillin-rich microfibrils. We present a model based on the structure described above that may explain how the cornea withstands repeated pressure changes due to the ocular pulse.
摘要: 角膜对可见光透明且具有精确的曲率, 以提供正确的折射能力。这两个特性都受其结构调控。角膜透明性是由于角膜基质中胶原纤维的相对有序排列, 使得可见光产生结构性干涉而形成的。这种排列受胶原纤维周围的带负电荷的蛋白多糖控制。对纤维组织的微小变化可耐受, 但较大的改变会导致光散射。角膜角质细胞不会使光散射, 因为它们的折射率与周围基质一致。但是, 当被激活时, 它们会变成折射率较低的成纤维细胞。建模显示, 这种变化会显著增加光的散射。在微观层面上, 角膜基质呈层状结构, 每层中的平行胶原纤维与其相邻层的纤维呈较大角度分布。X射线散射显示, 在人类角膜中这些片层具有优选方向: 角膜中央为上下、鼻-颞方向, 角膜缘为环形分布。角膜中央方向有助于抵御眼外肌的牵引, 而角膜缘处的伪圆形排列支持角膜与巩膜之间曲率的变化。弹性纤维也存在;在角膜缘, 它们含有围绕弹性蛋白核心的原纤维蛋白微纤维, 而在角膜中心, 它们以富含纤维蛋白的微纤维束的形式存在。我们提出了一个基于上述结构的模型, 该模型可以解释角膜如何乘受由于眼动脉搏动导致的反复压力变化。.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
-
- Dubbelman M, Sicam VA, Van der Heijde GL. The shape of the anterior and posterior surface of the aging human cornea. Vis Res. 2006;46:993–1001. - PubMed
-
- Patel S, Tutchenko L. The refractive index of the human cornea: a review. Cont Lens Anterior Eye. 2019;42:575–80. - PubMed
-
- Doughty MJ, Jonuscheit S. Corneal structure, transparency, thickness and optical density (densitometry), especially as relevant to contact lens wear-a review. Cont Lens Anterior Eye. 2019;42:238–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

