Dopamine projections to the basolateral amygdala drive the encoding of identity-specific reward memories
- PMID: 38396258
- PMCID: PMC11110430
- DOI: 10.1038/s41593-024-01586-7
Dopamine projections to the basolateral amygdala drive the encoding of identity-specific reward memories
Abstract
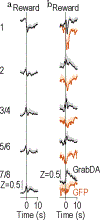
To make adaptive decisions, we build an internal model of the associative relationships in an environment and use it to make predictions and inferences about specific available outcomes. Detailed, identity-specific cue-reward memories are a core feature of such cognitive maps. Here we used fiber photometry, cell-type and pathway-specific optogenetic manipulation, Pavlovian cue-reward conditioning and decision-making tests in male and female rats, to reveal that ventral tegmental area dopamine (VTADA) projections to the basolateral amygdala (BLA) drive the encoding of identity-specific cue-reward memories. Dopamine is released in the BLA during cue-reward pairing; VTADA→BLA activity is necessary and sufficient to link the identifying features of a reward to a predictive cue but does not assign general incentive properties to the cue or mediate reinforcement. These data reveal a dopaminergic pathway for the learning that supports adaptive decision-making and help explain how VTADA neurons achieve their emerging multifaceted role in learning.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no biomedical financial interests or potential conflicts of interest.
Figures
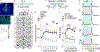



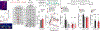




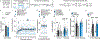

References
-
- Schultz W, Dayan P & Montague PR A neural substrate of prediction and reward. Science 275, 1593–1599 (1997). - PubMed
-
- Waelti P, Dickinson A & Schultz W Dopamine responses comply with basic assumptions of formal learning theory. Nature 412, 43–48 (2001). - PubMed
-
- Schultz W Predictive reward signal of dopamine neurons. J Neurophysiol 80, 1–27 (1998). - PubMed

