Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens
- PMID: 38396525
- PMCID: PMC10886341
- DOI: 10.3390/ani14040557
Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens
Abstract
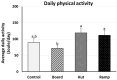
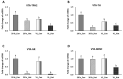
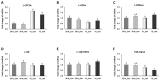
The aims of this study were to find suitable environmental enrichment (EE) and evaluate the combined effect of two EEs, variable light intensity (VL) lighting program and EH, on mental health and hepatic metabolic regulation in commercial broilers. To find the advantageous EEs for broilers, three different EEs (board, hut, and ramp) were tested in trial 1. EEs were placed and the engagement of birds to EEs, dustbathing behavior, and daily physical activity were observed. Birds treated with huts showed higher engagement than the board- or ramp-treated birds (p < 0.05). The results of dustbathing behavior and daily physical activity indicated that the environmental hut (EH) is the most favorable enrichment for broilers. In the second trial, to test the effect of EHs on mental health and hepatic metabolic conditions, the brain and liver were sampled from the four treatment birds (20 lx_Con, 20 lx_Hut, VL_Con and VL_Hut) on day 42. The lower expression of TPH2 (tryptophan hydroxylase 2) of VL_Hut birds than those of VL_Con and 20 lx_Hut treated birds suggests the combining effect of EHs with the VL lighting program on the central serotonergic homeostasis of broilers. Reduced expressions of TH (tyrosine hydroxylase), GR (glucocorticoid receptor), BDNF (brain-derived neurotrophic factor) of VL_Hut treated birds compared to those of VL_Con and 20 lx_Hut birds suggest lower stress, stress susceptibility, and chronic social stress in VL_Hut treated birds. The expression of CPT1A (carnitine palmitoyl transferase 1) increased over three-fold in the liver of VL_Con birds compared to 20 lx_Con birds (p < 0.05). EHs treatment in VL birds (VL_Hut) significantly decreased CPT1A but not in 20 lx birds (20 lx_Hut). The expression of ACCα (acetyl-CoA carboxylase alpha) was significantly decreased in VL_Con birds compared to 20 lx_Con birds. There was no significant difference in the hepatic FBPase (fructose-1,6-bisphosphatase), GR, and 11β-HSD1 (11 β-hydroxysteroid dehydrogenease-1) expression between 20 lx_Con and VL_Con birds, but EHs significantly stimulated GR in 20 lx_Hut birds, and stimulated FBPase and 11β-HSD1 expression in the VL_Hut birds compared to 20 lx_Con birds, suggesting that the VL lighting program reduced fatty acid synthesis and increased fatty acid β-oxidation in the broilers' liver and VL_Hut improved the hepatic de novo glucose production. Taken together, the results suggest that the stimulated voluntary activity by EHs in the light-enriched broiler house improved mental health and hepatic metabolic function of broilers and may indicate that the improved hepatic metabolic function contributes to efficient nutritional support for broilers.
Keywords: BDNF; dopamine; enrichment hut; hepatic fatty acid; serotonin; variable light intensity; welfare.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Dal Bosco A., Mattioli S., Cartoni Mancinelli A., Cotozzolo E., Castellini C. Extensive rearing systems in poultry production: The right chicken for the right farming system. A review of twenty years of scientific research in Perugia university, Italy. Animals. 2021;11:1281. doi: 10.3390/ani11051281. - DOI - PMC - PubMed
-
- Grzinic G., Piotrowicz-Cieslak A., Klimkowicz-Pawlas A., Gorny R.L., Lawniczek-Walczyk A., Piechowicz L., Olkowska E., Potrykus M., Tankiewicz M., Krupka M., et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci. Total. Environ. 2023;858:160014. doi: 10.1016/j.scitotenv.2022.160014. - DOI - PubMed
LinkOut - more resources
Full Text Sources

