Del-1 Plays a Protective Role against COPD Development by Inhibiting Inflammation and Apoptosis
- PMID: 38396634
- PMCID: PMC10888117
- DOI: 10.3390/ijms25041955
Del-1 Plays a Protective Role against COPD Development by Inhibiting Inflammation and Apoptosis
Abstract
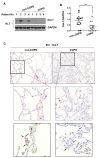
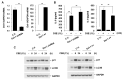
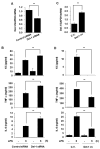
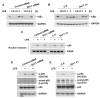
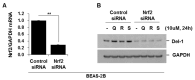
Neutrophilic inflammation is a prominent feature of chronic obstructive pulmonary disease (COPD). Developmental endothelial locus-1 (Del-1) has been reported to limit excessive neutrophilic inflammation by inhibiting neutrophil adhesion to the vascular endothelial cells. However, the effects of Del-1 in COPD are not known. We investigated the role of Del-1 in the pathogenesis of COPD. Del-1 protein expression was decreased in the lungs of COPD patients, especially in epithelial cells and alveolar macrophages. In contrast to human lung tissue, Del-1 expression was upregulated in lung tissue from mice treated with cigarette smoke extracts (CSE). Overexpression of Del-1 significantly suppressed IL-8 release and apoptosis in CSE-treated epithelial cells. In contrast, knockdown of Del-1 enhanced IL-8 release and apoptosis. In macrophages, overexpression of Del-1 significantly suppressed inflammatory cytokine release, and knockdown of Del-1 enhanced it. This anti-inflammatory effect was mediated by inhibiting the phosphorylation and acetylation of NF-κB p65. Nuclear factor erythroid 2-related factor 2 (Nrf2) activators, such as quercetin, resveratrol, and sulforaphane, increased Del-1 in both cell types. These results suggest that Del-1, mediated by Nrf2, plays a protective role against the pathogenesis of COPD, at least in part through anti-inflammatory and anti-apoptotic effects.
Keywords: COPD; Del-1; Nrf2; apoptosis; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Adeloye D., Song P., Zhu Y., Campbell H., Sheikh A., Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. - DOI - PMC - PubMed
-
- Safiri S., Carson-Chahhoud K., Noori M., Nejadghaderi S.A., Sullman M.J., Heris J.A., Ansarin K., Mansournia M.A., Collins G.S., Kolahi A.-A. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679. doi: 10.1136/bmj-2021-069679. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

