Antineoplastic Activity of 9″-Lithospermic Acid Methyl Ester in Glioblastoma Cells
- PMID: 38396771
- PMCID: PMC10889145
- DOI: 10.3390/ijms25042094
Antineoplastic Activity of 9″-Lithospermic Acid Methyl Ester in Glioblastoma Cells
Abstract
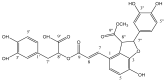
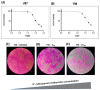
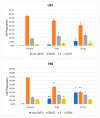
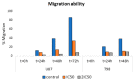
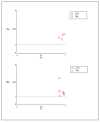
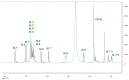
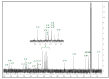
To date, many potent compounds have been found which are derived from plants and herbs and possess anticancer properties due to their antioxidant effects. 9″-Lithospermic acid methyl ester is an effective natural compound derived from the Thymus thracicus Velen. It has been proven that this compound has substantial properties in different diseases, but its effects in cancer have not been thoroughly evaluated. The aim of this work was to study the effects of 9″-Lithospermic acid methyl ester (9″-methyl lithospermate) in U87 and T98 glioblastoma cell lines. Its effects on cellular viability were assessed via Trypan Blue and Crystal Violet stains, the cell cycle analysis through flow cytometry, and cell migration by employing the scratch wound healing assay. The results demonstrated that 9″-methyl lithospermate was able to inhibit cellular proliferation, induce cellular death, and inhibit cell migration. Furthermore, these results were intensified by the addition of temozolomide, the most prominent chemotherapeutic drug in glioblastoma tumors. Further studies are needed to reproduce these findings in animal models and investigate if 9″-lithospermic acid methyl ester represents a potential new therapeutic addition for gliomas.
Keywords: 9″-lithospermic acid methyl ester; cancer; glioblastoma; gliomas; natural products; temozolomide.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Nabors L.B., Portnow J., Ahluwalia M., Baehring J., Brem H., Brem S., Butowski N., Campian J.L., Clark S.W., Fabiano A.J., et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020;18:1537–1570. doi: 10.6004/jnccn.2020.0052. - DOI - PubMed
-
- Tamimi A.F., Juweid M. Glioblastoma [Internet] Codon Publications; Brisbane, Australia: 2017. Epidemiology and Outcome of Glioblastoma; pp. 143–153. - PubMed
-
- Ostrom Q.T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N.E., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15((Suppl. 2)):ii1–ii56. doi: 10.1093/neuonc/not151. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

