A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody-Drug Conjugates
- PMID: 38396776
- PMCID: PMC10889805
- DOI: 10.3390/ijms25042097
A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody-Drug Conjugates
Abstract
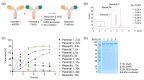
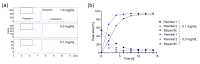
The toolbox of modern antibody engineering allows the design of versatile novel functionalities exceeding nature's repertoire. Many bispecific antibodies comprise heterodimeric Fc portions recently validated through the approval of several bispecific biotherapeutics. While heterodimerization methodologies have been established for low-throughput large-scale production, few approaches exist to overcome the bottleneck of large combinatorial screening efforts that are essential for the identification of the best possible bispecific antibody. This report presents a novel, robust and miniaturized heterodimerization process based on controlled Fab-arm exchange (cFAE), which is applicable to a variety of heterodimeric formats and compatible with automated high-throughput screens. Proof of applicability was shown for two therapeutic molecule classes and two relevant functional screening read-outs. First, the miniaturized production of biparatopic anti-c-MET antibody-drug conjugates served as a proof of concept for their applicability in cytotoxic screenings on tumor cells with different target expression levels. Second, the automated workflow enabled a large unbiased combinatorial screening of biparatopic antibodies and the identification of hits mediating potent c-MET degradation. The presented workflow utilizes standard equipment and may serve as a facile, efficient and robust method for the discovery of innovative therapeutic agents in many laboratories worldwide.
Keywords: DuoBody; automation; bispecific antibody; miniaturization; unbiased screening.
Conflict of interest statement
All authors are or were employees of Merck KGaA/EMD Serono Inc. Besides this, the authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

