Current Understanding of Immune Thrombocytopenia: A Review of Pathogenesis and Treatment Options
- PMID: 38396839
- PMCID: PMC10889445
- DOI: 10.3390/ijms25042163
Current Understanding of Immune Thrombocytopenia: A Review of Pathogenesis and Treatment Options
Abstract
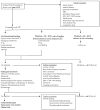
The management of immune thrombocytopenia (ITP) and the prediction of patient response to therapy still represent a significant and constant challenge in hematology. ITP is a heterogeneous disease with an unpredictable evolution. Although the pathogenesis of ITP is currently better known and its etiology has been extensively studied, up to 75% of adult patients with ITP may develop chronicity, which represents a significant burden on patients' quality of life. A major risk of ITP is bleeding, but knowledge on the exact relationship between the degree of thrombocytopenia and bleeding symptoms, especially at a lower platelet count, is lacking. The actual management of ITP is based on immune suppression (corticosteroids and intravenous immunoglobulins), or the use of thrombopoietin receptor agonists (TPO-RAs), rituximab, or spleen tyrosine kinase (Syk) inhibitors. A better understanding of the underlying pathology has facilitated the development of a number of new targeted therapies (Bruton's tyrosine kinase inhibitors, neonatal Fc receptors, strategies targeting B and plasma cells, strategies targeting T cells, complement inhibitors, and newer TPO-RAs for improving megakaryopoiesis), which seem to be highly effective and well tolerated and result in a significant improvement in patients' quality of life. The disadvantage is that there is a lack of knowledge of the predictive factors of response to treatments, which would help in the development of an optimized treatment algorithm for selected patients.
Keywords: ITP; SYK inhibitors; TPO-RA; pathogenesis; platelet desialylation; rituximab; splenectomy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D.M., Bussel J.B., Cines D.B., Chong B.H., Cooper N., et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

