Fibroblasts in Diabetic Foot Ulcers
- PMID: 38396848
- PMCID: PMC10889208
- DOI: 10.3390/ijms25042172
Fibroblasts in Diabetic Foot Ulcers
Abstract
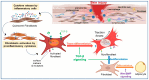
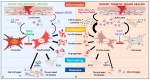
Fibroblasts are stromal cells ubiquitously distributed in the body of nearly every organ tissue. These cells were previously considered to be "passive cells", solely responsible for ensuring the turnover of the extracellular matrix (ECM). However, their versatility, including their ability to switch phenotypes in response to tissue injury and dynamic activity in the maintenance of tissue specific homeostasis and integrity have been recently revealed by the innovation of technological tools such as genetically modified mouse models and single cell analysis. These highly plastic and heterogeneous cells equipped with multifaceted functions including the regulation of angiogenesis, inflammation as well as their innate stemness characteristics, play a central role in the delicately regulated process of wound healing. Fibroblast dysregulation underlies many chronic conditions, including cardiovascular diseases, cancer, inflammatory diseases, and diabetes mellitus (DM), which represent the current major causes of morbidity and mortality worldwide. Diabetic foot ulcer (DFU), one of the most severe complications of DM affects 40 to 60 million people. Chronic non-healing DFU wounds expose patients to substantial sequelae including infections, gangrene, amputation, and death. A complete understanding of the pathophysiology of DFU and targeting pathways involved in the dysregulation of fibroblasts are required for the development of innovative new therapeutic treatments, critically needed for these patients.
Keywords: chronic inflammation; chronic wound; diabetic foot ulcer; diabetic wound; fibroblast; myofibroblast.
Conflict of interest statement
Carlos Theodore Huerta, Nga Le, Hongwei Shao, Yuli Ortiz and Francesca Voza have no commercial or financial relationships that could be construed as a potential conflict of interest. Dr. Zhao-Jun Liu (Z.-J.L.) and Dr. Omaida C. Velazquez (O.C.V.) declare the following potential conflicts of interest with respect to the research, authorship, and/or presentation and/or publication of some aspects of this work: the E-selectin gene modification technologies were developed in our research laboratory and patented/licensed by the University of Miami. This technology is currently under pre-clinical development by Ambulero Inc., a new start-up company out of the University of Miami that focuses on developing new vascular treatments for ischemic tissue conditions and limb salvage. Co-authors, Z.-J.L. and O.C.V., serve as consultants and chief scientific and medical advisory officers, are co-Inventors of the technologies, and are minority shareholders in Ambulero Inc. Co-authors, Z.-J.L. and O.C.V. are also funded by the NIH/NHLBI and philanthropy in preclinical investigations of these technologies.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

