MicroRNA205: A Key Regulator of Cardiomyocyte Transition from Proliferative to Hypertrophic Growth in the Neonatal Heart
- PMID: 38396885
- PMCID: PMC10889831
- DOI: 10.3390/ijms25042206
MicroRNA205: A Key Regulator of Cardiomyocyte Transition from Proliferative to Hypertrophic Growth in the Neonatal Heart
Abstract
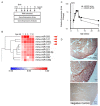
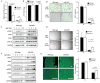
The mammalian myocardium grows rapidly during early development due to cardiomyocyte proliferation, which later transitions to cell hypertrophy to sustain the heart's postnatal growth. Although this cell transition in the postnatal heart is consistently preserved in mammalian biology, little is known about the regulatory mechanisms that link proliferation suppression with hypertrophy induction. We reasoned that the production of a micro-RNA(s) could serve as a key bridge to permit changes in gene expression that control the changed cell fate of postnatal cardiomyocytes. We used sequential expression analysis to identify miR205 as a micro-RNA that was uniquely expressed at the cessation of cardiomyocyte growth. Cardiomyocyte-specific miR205 deletion animals showed a 35% increase in heart mass by 3 months of age, with commensurate changes in cell cycle and Hippo pathway activity, confirming miR205's potential role in controlling cardiomyocyte proliferation. In contrast, overexpression of miR205 in newborn hearts had little effect on heart size or function, indicating a complex, probably redundant regulatory system. These findings highlight miR205's role in controlling the shift from cardiomyocyte proliferation to hypertrophic development in the postnatal period.
Keywords: Hippo pathway; hypertrophy; postnatal heart development.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
A specialized population of Periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation.Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21469-21479. doi: 10.1073/pnas.2009119117. Epub 2020 Aug 17. Proc Natl Acad Sci U S A. 2020. PMID: 32817558 Free PMC article.
-
mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts.Circ Res. 2013 Jun 7;112(12):1557-66. doi: 10.1161/CIRCRESAHA.112.300658. Epub 2013 Apr 10. Circ Res. 2013. PMID: 23575307 Free PMC article.
-
Regulatory Mechanisms That Guide the Fetal to Postnatal Transition of Cardiomyocytes.Cells. 2023 Sep 21;12(18):2324. doi: 10.3390/cells12182324. Cells. 2023. PMID: 37759546 Free PMC article. Review.
-
RNA-Binding Protein LIN28a Regulates New Myocyte Formation in the Heart Through Long Noncoding RNA-H19.Circulation. 2023 Jan 24;147(4):324-337. doi: 10.1161/CIRCULATIONAHA.122.059346. Epub 2022 Oct 31. Circulation. 2023. PMID: 36314132 Free PMC article.
-
Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration?Circ Res. 2020 Feb 14;126(4):552-565. doi: 10.1161/CIRCRESAHA.119.315408. Epub 2020 Feb 13. Circ Res. 2020. PMID: 32078450 Review.
Cited by
-
Recent Clinical Updates of Hypertrophic Cardiomyopathy and Future Therapeutic Strategies.Rev Cardiovasc Med. 2025 Feb 20;26(2):25132. doi: 10.31083/RCM25132. eCollection 2025 Feb. Rev Cardiovasc Med. 2025. PMID: 40026515 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

