Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation
- PMID: 38396887
- PMCID: PMC10889495
- DOI: 10.3390/ijms25042210
Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation
Abstract
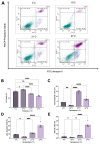
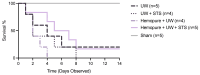
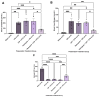
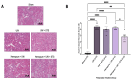
Kidney transplantation is preferred for end-stage renal disease. The current gold standard for kidney preservation is static cold storage (SCS) at 4 °C. However, SCS contributes to renal graft damage through ischemia-reperfusion injury (IRI). We previously reported renal graft protection after SCS with a hydrogen sulfide donor, sodium thiosulfate (STS), at 4 °C. Therefore, this study aims to investigate whether SCS at 10 °C with STS and Hemopure (blood substitute), will provide similar protection. Using in vitro model of IRI, we subjected rat renal proximal tubular epithelial cells to hypoxia-reoxygenation for 24 h at 10 °C with or without STS and measured cell viability. In vivo, we preserved 36 donor kidneys of Lewis rats for 24 h in a preservation solution at 10 °C supplemented with STS, Hemopure, or both followed by transplantation. Tissue damage and recipient graft function parameters, including serum creatinine, blood urea nitrogen, urine osmolality, and glomerular filtration rate (GFR), were evaluated. STS-treated proximal tubular epithelial cells exhibited enhanced viability at 10 °C compared with untreated control cells (p < 0.05). Also, STS and Hemopure improved renal graft function compared with control grafts (p < 0.05) in the early time period after the transplant, but long-term function did not reach significance. Overall, renal graft preservation at 10 °C with STS and Hemopure supplementation has the potential to enhance graft function and reduce kidney damage, suggesting a novel approach to reducing IRI and post-transplant complications.
Keywords: Hemopure; graft and recipient survival; ischemia–reperfusion injury (IRI); kidney transplantation; sodium thiosulfate (STS); static cold storage (SCS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation.Int J Mol Sci. 2024 Sep 2;25(17):9529. doi: 10.3390/ijms25179529. Int J Mol Sci. 2024. PMID: 39273476 Free PMC article.
-
Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation.Biomed Pharmacother. 2022 Jan;145:112435. doi: 10.1016/j.biopha.2021.112435. Epub 2021 Nov 17. Biomed Pharmacother. 2022. PMID: 34798469
-
Supplemental hydrogen sulphide protects transplant kidney function and prolongs recipient survival after prolonged cold ischaemia-reperfusion injury by mitigating renal graft apoptosis and inflammation.BJU Int. 2012 Dec;110(11 Pt C):E1187-95. doi: 10.1111/j.1464-410X.2012.11526.x. Epub 2012 Nov 16. BJU Int. 2012. PMID: 23157304
-
The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation.Int J Mol Sci. 2022 Dec 29;24(1):567. doi: 10.3390/ijms24010567. Int J Mol Sci. 2022. PMID: 36614006 Free PMC article. Review.
-
Pre-Treatment of Transplant Donors with Hydrogen Sulfide to Protect against Warm and Cold Ischemia-Reperfusion Injury in Kidney and Other Transplantable Solid Organs.Int J Mol Sci. 2023 Feb 9;24(4):3518. doi: 10.3390/ijms24043518. Int J Mol Sci. 2023. PMID: 36834928 Free PMC article. Review.
Cited by
-
Opportunities and challenges with the implementation of normothermic machine perfusion in kidney transplantation.Nat Commun. 2025 Jul 25;16(1):6883. doi: 10.1038/s41467-025-60410-3. Nat Commun. 2025. PMID: 40715058 Free PMC article. Review.
-
Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation.Int J Mol Sci. 2024 Sep 2;25(17):9529. doi: 10.3390/ijms25179529. Int J Mol Sci. 2024. PMID: 39273476 Free PMC article.
-
Molecular Mechanisms and Potential Therapeutic Targets of Ischemia-Reperfusion Injury in Kidney Transplantation.Curr Issues Mol Biol. 2025 Apr 17;47(4):282. doi: 10.3390/cimb47040282. Curr Issues Mol Biol. 2025. PMID: 40699682 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

