Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors
- PMID: 38396906
- PMCID: PMC10889403
- DOI: 10.3390/ijms25042231
Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors
Abstract
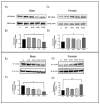
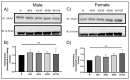
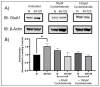
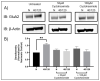
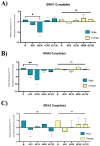
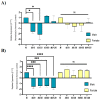
Following ischemia/reperfusion, AMPA receptors (AMPARs) mediate pathologic delayed neuronal death through sustained expression of calcium-permeable AMPARs, leading to excitotoxicity. Preventing the surface removal of GluA2-containing AMPARs may yield new therapeutic targets for the treatment of ischemia/reperfusion. This study utilized acute organotypic hippocampal slices from aged male and female Sprague Dawley rats and subjected them to oxygen-glucose deprivation/reperfusion (OGD/R) to examine the mechanisms underlying the internalization and degradation of GluA2-containing AMPARs. We determined the effect of OGD/R on AMPAR subunits at the protein and mRNA transcript levels utilizing Western blot and RT-qPCR, respectively. Hippocampal slices from male and female rats responded to OGD/R in a paradoxical manner with respect to AMPARs. GluA1 and GluA2 AMPAR subunits were degraded following OGD/R in male rats but were increased in female rats. There was a rapid decrease in GRIA1 (GluA1) and GRIA2 (GluA2) mRNA levels in the male hippocampus following ischemic insult, but this was not observed in females. These data indicate a sex-dependent difference in how AMPARs in the hippocampus respond to ischemic insult, and may help explain, in part, why premenopausal women have a lower incidence/severity of ischemic stroke compared with men of the same age.
Keywords: AMPA receptor; GluA2; degradation; hippocampus; ischemic/reperfusion injury; mRNA; oxygen glucose deprivation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Oxidative Stress Underlies the Ischemia/Reperfusion-Induced Internalization and Degradation of AMPA Receptors.Int J Mol Sci. 2021 Jan 13;22(2):717. doi: 10.3390/ijms22020717. Int J Mol Sci. 2021. PMID: 33450848 Free PMC article.
-
Identification of NADPH oxidase as a key mediator in the post-ischemia-induced sequestration and degradation of the GluA2 AMPA receptor subunit.J Neurochem. 2015 Mar;132(5):504-19. doi: 10.1111/jnc.13005. Epub 2015 Feb 4. J Neurochem. 2015. PMID: 25475532
-
Endocytosis and lysosomal degradation of GluA2/3 AMPARs in response to oxygen/glucose deprivation in hippocampal but not cortical neurons.Sci Rep. 2017 Sep 26;7(1):12318. doi: 10.1038/s41598-017-12534-w. Sci Rep. 2017. PMID: 28951554 Free PMC article.
-
Distinct subunit-specific α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking mechanisms in cultured cortical and hippocampal neurons in response to oxygen and glucose deprivation.J Biol Chem. 2014 Feb 21;289(8):4644-51. doi: 10.1074/jbc.M113.533182. Epub 2014 Jan 8. J Biol Chem. 2014. PMID: 24403083 Free PMC article.
-
The AMPAR subunit GluR2: still front and center-stage.Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
References
-
- Marier J. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1587. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

