Association of MDM2 Overexpression in Ameloblastomas with MDM2 Amplification and BRAFV600E Expression
- PMID: 38396916
- PMCID: PMC10889355
- DOI: 10.3390/ijms25042238
Association of MDM2 Overexpression in Ameloblastomas with MDM2 Amplification and BRAFV600E Expression
Abstract
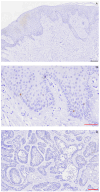
Ameloblastoma is a rare tumor but represents the most common odontogenic neoplasm. It is localized in the jaws and, although it is a benign, slow-growing tumor, it has an aggressive local behavior and high recurrence rate. Therefore, alternative treatment options or complementary to surgery have been evaluated, with the most promising one among them being a targeted therapy with the v-Raf murine sarcoma viral oncogene homologue B (BRAF), as in ameloblastoma the activating mutation V600E in BRAF is common. Studies in other tumors have shown that the synchronous inhibition of BRAF and human murine double minute 2 homologue (MDM2 or HDM2) protein is more effective than BRAF monotherapy, particularly in the presence of wild type p53 (WTp53). To investigate the MDM2 protein expression and gene amplification in ameloblastoma, in association with BRAFV600E and p53 expression. Forty-four cases of ameloblastoma fixed in 10% buffered formalin and embedded in paraffin were examined for MDM2 overexpression and BRAFV600E and p53 expression by immunohistochemistry, and for MDM2 ploidy with fluorescence in situ hybridization. Sixteen of forty-four (36.36%) cases of ameloblastoma showed MDM2 overexpression. Seven of sixteen MDM2-positive ameloblastomas (43.75%) were BRAFV600E positive and fifteen of sixteen MDM2-positive ameloblastomas (93.75%) were p53 negative. All MDM2 overexpressing tumors did not show copy number alterations for MDM2. Overexpression of MDM2 in ameloblastomas is not associated with MDM2 amplification, but most probably with MAPK activation and WTp53 expression. Further verification of those findings could form the basis for the use of MDM2 expression as a marker of MAPK activation in ameloblastomas and the trial of dual BRAF/MDM2 inhibition in the management of MDM2-overexpressing/BRAFV600E-positive/WTp53 ameloblastomas.
Keywords: BRAF; MDM2 protein; ameloblastoma; fluorescence; in situ hybridization; odontogenic tumors; p53.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
p53 gene status and expression of p53, MDM2, and p14 proteins in ameloblastomas.J Oral Pathol Med. 2004 May;33(5):292-9. doi: 10.1111/j.0904-2512.2004.00044.x. J Oral Pathol Med. 2004. PMID: 15078490
-
High frequency of BRAF V600E mutation in Iranian population ameloblastomas.Med Oral Patol Oral Cir Bucal. 2020 Jul 1;25(4):e502-e507. doi: 10.4317/medoral.23519. Med Oral Patol Oral Cir Bucal. 2020. PMID: 32388526 Free PMC article.
-
Detection and evaluation of the presence of the BRAF V600E mutation in ameloblastomas in an Indian population.Indian J Pathol Microbiol. 2023 Apr-Jun;66(2):246-251. doi: 10.4103/ijpm.ijpm_398_21. Indian J Pathol Microbiol. 2023. PMID: 37077063
-
Diagnostic accuracy of immunohistochemistry compared with molecular tests for detection of BRAF V600E mutation in ameloblastomas: Systematic review and meta-analysis.J Oral Pathol Med. 2022 Mar;51(3):223-230. doi: 10.1111/jop.13278. Epub 2022 Feb 8. J Oral Pathol Med. 2022. PMID: 35090195
-
Frequency of BRAF V600E immunoexpression in ameloblastomas: a multi-institutional analysis of 86 cases in Latin America and comprehensive review of the literature.Med Oral Patol Oral Cir Bucal. 2024 Jul 1;29(4):e509-e516. doi: 10.4317/medoral.26493. Med Oral Patol Oral Cir Bucal. 2024. PMID: 38615253 Free PMC article. Review.
Cited by
-
Unlocking the Gateway: The Spatio-Temporal Dynamics of the p53 Family Driven by the Nuclear Pores and Its Implication for the Therapeutic Approach in Cancer.Int J Mol Sci. 2024 Jul 7;25(13):7465. doi: 10.3390/ijms25137465. Int J Mol Sci. 2024. PMID: 39000572 Free PMC article. Review.
-
Immunohistochemical Expression of MDM2, Bcl-2, SATB2 and Ki-67 in Histological Variants of Unicystic Ameloblastoma.Head Neck Pathol. 2024 Oct 15;18(1):100. doi: 10.1007/s12105-024-01705-7. Head Neck Pathol. 2024. PMID: 39404986
References
-
- Siriwardena B., Crane H., O’Neill N., Abdelkarim R., Brierley D.J., Franklin C.D., Farthing P.M., Speight P.M., Hunter K.D. Odontogenic tumors and lesions treated in a single specialist oral and maxillofacial pathology unit in the United Kingdom in 1992–2016. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019;127:151–166. doi: 10.1016/j.oooo.2018.09.011. - DOI - PubMed
-
- Boffano P., Cavarra F., Tricarico G., Masu L., Brucoli M., Ruslin M., Forouzanfar T., Ridwan-Pramana A., Rodriguez-Santamarta T., Rui Ranz M., et al. The epidemiology and management of ameloblastomas: A European multicenter study. J. Craniomaxillofac. Surg. 2021;49:1107–1112. doi: 10.1016/j.jcms.2021.09.007. - DOI - PubMed
-
- El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J. WHO Classification of Head and Neck Tumours. 4th ed. IARC; Lyon, France: 2017. Odontogenic and maxillofacial bone tumors; pp. 215–219.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

