Long-Term Outcomes after Switching to Tenofovir Alafenamide in Patients with Chronic Hepatitis B
- PMID: 38396921
- PMCID: PMC10888772
- DOI: 10.3390/ijms25042245
Long-Term Outcomes after Switching to Tenofovir Alafenamide in Patients with Chronic Hepatitis B
Abstract
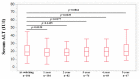
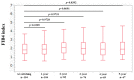
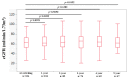
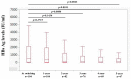
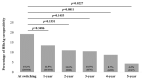
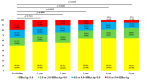
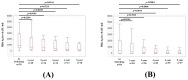
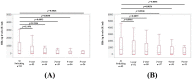
We sought to determine the long-term outcomes of chronic hepatitis B (CHB) cases switching to tenofovir alafenamide (TAF, n = 104, median age = 63.5 years). Data at switching to TAF (baseline) and those at 1, 2, 3, 4, and 5 years from switching to TAF were compared. At baseline, HB envelop antigen (HBeAg) seropositivity was found in 20 patients (19.2%), and undetectable HBV-DNA in 77 patients (74.0%). Percentage of detectable HBV-DNA significantly reduced at any time point. HB surface antigen (HBsAg) levels significantly reduced at 3, 4, and 5 years. The percentage of HBeAg seropositivity significantly reduced at 5 years. HB core related antigen levels did not significantly change. In patients with baseline HbeAg seropositivity, HbsAg levels significantly reduced at any time point, and a similar trend was found in patients without HBeAg seropositivity. In patients with baseline FIB4 index >1.85, HBsAg levels significantly reduced at 3, 4, and 5 years, and in patients with baseline FIB4 index <1.85, HBsAg levels significantly reduced at any time point. The estimated glomerular filtration rate significantly reduced only at 5 years. The discontinuation rate owing to the side effects of TAF was 0%. In conclusion, switching to TAF therapy in patients with CHB may be effective and safe at least up to 5 years.
Keywords: chronic hepatitis B; efficacy; safety; switch; tenofovir alafenamide.
Conflict of interest statement
None of the authors has any conflicts of interest to declare.
Figures

Similar articles
-
Prospective Analysis of Safety and Efficacy of Tenofovir Alafenamide Fumarate (TAF) in European Real-World Patients with Chronic Hepatitis B: A Single-Centre Real-Word Cohort Study.Pathogens. 2024 Sep 23;13(9):820. doi: 10.3390/pathogens13090820. Pathogens. 2024. PMID: 39339010 Free PMC article.
-
Comparative efficacy and safety of tenofovir amibufenamide vs tenofovir alafenamide in the initial 48-week treatment of high viral load chronic hepatitis B: A single-centre retrospective study.Antivir Ther. 2024 Oct;29(5):13596535241284226. doi: 10.1177/13596535241284226. Antivir Ther. 2024. PMID: 39259839
-
[Clinical efficacy analysis of TMF for the treatment of hyperviremia HBeAg-positive chronic hepatitis B patients with incomplete response to first-line oral antiviral nucleos(t)ide analogues].Zhonghua Gan Zang Bing Za Zhi. 2023 Mar 20;31(3):252-257. doi: 10.3760/cma.j.cn501113-20230212-00052. Zhonghua Gan Zang Bing Za Zhi. 2023. PMID: 37137850 Chinese.
-
Tenofovir alafenamide for the treatment of chronic hepatitis B virus infection.Expert Rev Clin Pharmacol. 2017 Jul;10(7):707-716. doi: 10.1080/17512433.2017.1323633. Epub 2017 May 17. Expert Rev Clin Pharmacol. 2017. PMID: 28460547 Review.
-
Tenofovir alafenamide as compared to tenofovir disoproxil fumarate in the management of chronic hepatitis B with recent trends in patient demographics.Expert Rev Gastroenterol Hepatol. 2017 Nov;11(11):999-1008. doi: 10.1080/17474124.2017.1386554. Epub 2017 Oct 8. Expert Rev Gastroenterol Hepatol. 2017. PMID: 28965428 Review.
Cited by
-
Prospective Analysis of Safety and Efficacy of Tenofovir Alafenamide Fumarate (TAF) in European Real-World Patients with Chronic Hepatitis B: A Single-Centre Real-Word Cohort Study.Pathogens. 2024 Sep 23;13(9):820. doi: 10.3390/pathogens13090820. Pathogens. 2024. PMID: 39339010 Free PMC article.
References
-
- Lampertico P., Buti M., Fung S., Ahn S.H., Chuang W.L., Tak W.Y., Ramji A., Chen C.Y., Tam E., Bae H., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: A randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol. Hepatol. 2020;5:441–453. doi: 10.1016/S2468-1253(19)30421-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

