Long-Term Outcomes after Switching to Tenofovir Alafenamide in Patients with Chronic Hepatitis B
- PMID: 38396921
- PMCID: PMC10888772
- DOI: 10.3390/ijms25042245
Long-Term Outcomes after Switching to Tenofovir Alafenamide in Patients with Chronic Hepatitis B
Abstract
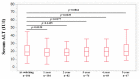
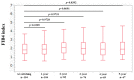
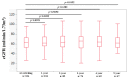
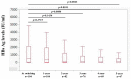
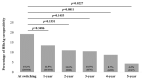
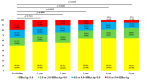
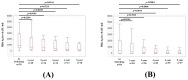
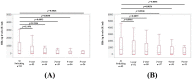
We sought to determine the long-term outcomes of chronic hepatitis B (CHB) cases switching to tenofovir alafenamide (TAF, n = 104, median age = 63.5 years). Data at switching to TAF (baseline) and those at 1, 2, 3, 4, and 5 years from switching to TAF were compared. At baseline, HB envelop antigen (HBeAg) seropositivity was found in 20 patients (19.2%), and undetectable HBV-DNA in 77 patients (74.0%). Percentage of detectable HBV-DNA significantly reduced at any time point. HB surface antigen (HBsAg) levels significantly reduced at 3, 4, and 5 years. The percentage of HBeAg seropositivity significantly reduced at 5 years. HB core related antigen levels did not significantly change. In patients with baseline HbeAg seropositivity, HbsAg levels significantly reduced at any time point, and a similar trend was found in patients without HBeAg seropositivity. In patients with baseline FIB4 index >1.85, HBsAg levels significantly reduced at 3, 4, and 5 years, and in patients with baseline FIB4 index <1.85, HBsAg levels significantly reduced at any time point. The estimated glomerular filtration rate significantly reduced only at 5 years. The discontinuation rate owing to the side effects of TAF was 0%. In conclusion, switching to TAF therapy in patients with CHB may be effective and safe at least up to 5 years.
Keywords: chronic hepatitis B; efficacy; safety; switch; tenofovir alafenamide.
Conflict of interest statement
None of the authors has any conflicts of interest to declare.
Figures

References
-
- Lampertico P., Buti M., Fung S., Ahn S.H., Chuang W.L., Tak W.Y., Ramji A., Chen C.Y., Tam E., Bae H., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: A randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol. Hepatol. 2020;5:441–453. doi: 10.1016/S2468-1253(19)30421-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

