The Metabolic Syndrome, a Human Disease
- PMID: 38396928
- PMCID: PMC10888680
- DOI: 10.3390/ijms25042251
The Metabolic Syndrome, a Human Disease
Abstract
This review focuses on the question of metabolic syndrome (MS) being a complex, but essentially monophyletic, galaxy of associated diseases/disorders, or just a syndrome of related but rather independent pathologies. The human nature of MS (its exceptionality in Nature and its close interdependence with human action and evolution) is presented and discussed. The text also describes the close interdependence of its components, with special emphasis on the description of their interrelations (including their syndromic development and recruitment), as well as their consequences upon energy handling and partition. The main theories on MS's origin and development are presented in relation to hepatic steatosis, type 2 diabetes, and obesity, but encompass most of the MS components described so far. The differential effects of sex and its biological consequences are considered under the light of human social needs and evolution, which are also directly related to MS epidemiology, severity, and relations with senescence. The triggering and maintenance factors of MS are discussed, with especial emphasis on inflammation, a complex process affecting different levels of organization and which is a critical element for MS development. Inflammation is also related to the operation of connective tissue (including the adipose organ) and the widely studied and acknowledged influence of diet. The role of diet composition, including the transcendence of the anaplerotic maintenance of the Krebs cycle from dietary amino acid supply (and its timing), is developed in the context of testosterone and β-estradiol control of the insulin-glycaemia hepatic core system of carbohydrate-triacylglycerol energy handling. The high probability of MS acting as a unique complex biological control system (essentially monophyletic) is presented, together with additional perspectives/considerations on the treatment of this 'very' human disease.
Keywords: adipose organ; connective tissue; dietary nutrient handling; energy partition; inflammation; insulin; metabolic syndrome; protein-fueled anaplerosis; testosterone; β estradiol.
Conflict of interest statement
The author declares no conflicts of interests.
Figures
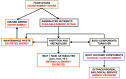

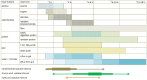

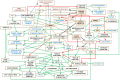
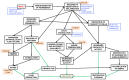


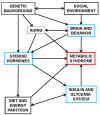
References
-
- Hanefeld M., Leonhardt W. Das metabolische syndrom. Dtsch. Gesundheitsw. Z. Klin. Med. 1981;36:545–551. doi: 10.1055/s-2006-947258. - DOI
-
- Chopra A.K. Metabolic syndrome or insulin resistance: Evolution, controversies and association with cardiovascular disease risk. Indian J. Clin. Cardiol. 2020;1:77–85. doi: 10.1177/2632463620935030. - DOI
-
- Alemany M. Metabolic syndrome: A multifaceted disease of affluence. J. Endocrinol. Metab. 2012;2:155–165. doi: 10.4021/jem116w. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

