Integrating Continuous Transepithelial Flux Measurements into an Ussing Chamber Set-Up
- PMID: 38396929
- PMCID: PMC10889482
- DOI: 10.3390/ijms25042252
Integrating Continuous Transepithelial Flux Measurements into an Ussing Chamber Set-Up
Abstract
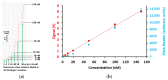
Fluorescently labelled compounds are often employed to study the paracellular properties of epithelia. For flux measurements, these compounds are added to the donor compartment and samples collected from the acceptor compartment at regular intervals. However, this method fails to detect rapid changes in permeability. For continuous transepithelial flux measurements in an Ussing chamber setting, a device was developed, consisting of a flow-through chamber with an attached LED, optical filter, and photodiode, all encased in a light-impermeable container. The photodiode output was amplified and recorded. Calibration with defined fluorescein concentration (range of 1 nM to 150 nM) resulted in a linear output. As proof of principle, flux measurements were performed on various cell lines. The results confirmed a linear dependence of the flux on the fluorescein concentration in the donor compartment. Flux depended on paracellular barrier function (expression of specific tight junction proteins, and EGTA application to induce barrier loss), whereas activation of transcellular chloride secretion had no effect on fluorescein flux. Manipulation of the lateral space by osmotic changes in the perfusion solution also affected transepithelial fluorescein flux. In summary, this device allows a continuous recording of transepithelial flux of fluorescent compounds in parallel with the electrical parameters recorded by the Ussing chamber.
Keywords: Ussing chamber; automatized flux measurement; claudin; osmotic stress; paracellular fluorescent marker; tight junction; transepithelial flux.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Laurate permeabilizes the paracellular pathway for small molecules in the intestinal epithelial cell model HT-29/B6 via opening the tight junctions by reversible relocation of claudin-5. [Corrected].Pharm Res. 2014 Sep;31(9):2539-48. doi: 10.1007/s11095-014-1350-2. Epub 2014 Mar 15. Pharm Res. 2014. PMID: 24633418
-
Cholera toxin perturbs the paracellular barrier in the small intestinal epithelium of rats by affecting claudin-2 and tricellulin.Pflugers Arch. 2019 Sep;471(9):1183-1189. doi: 10.1007/s00424-019-02294-z. Epub 2019 Jun 20. Pflugers Arch. 2019. PMID: 31222489
-
Loss of NKCC1 function increases epithelial tight junction permeability by upregulating claudin-2 expression.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C1251-C1263. doi: 10.1152/ajpcell.00334.2022. Epub 2022 Aug 15. Am J Physiol Cell Physiol. 2022. PMID: 35968893 Free PMC article.
-
Beyond Ussing's chambers: contemporary thoughts on integration of transepithelial transport.Am J Physiol Cell Physiol. 2016 Mar 15;310(6):C423-31. doi: 10.1152/ajpcell.00348.2015. Epub 2015 Dec 23. Am J Physiol Cell Physiol. 2016. PMID: 26702131 Free PMC article. Review.
-
[Claudins as tight junction proteins: the molecular element of paracellular transport].Ross Fiziol Zh Im I M Sechenova. 2013 Feb;99(2):175-95. Ross Fiziol Zh Im I M Sechenova. 2013. PMID: 23650732 Review. Russian.
Cited by
-
Special Issue "The Tight Junction and Its Proteins: From Structure to Pathologies".Int J Mol Sci. 2024 Dec 7;25(23):13154. doi: 10.3390/ijms252313154. Int J Mol Sci. 2024. PMID: 39684864 Free PMC article.
-
Ion permeability profiles of renal paracellular channel-forming claudins.Acta Physiol (Oxf). 2025 Feb;241(2):e14264. doi: 10.1111/apha.14264. Acta Physiol (Oxf). 2025. PMID: 39821681 Free PMC article.
References
-
- Dittmann I., Amasheh M., Krug S.M., Markov A.G., Fromm M., Amasheh S. Laurate permeabilizes the paracellular pathway for small molecules in the intestinal epithelial cell model HT-29/B6 via opening the tight junctions by reversible relocation of claudin-5. [Corrected] Pharm Res. 2014;31:2539–2548. doi: 10.1007/s11095-014-1350-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

