Identification of Hit Compounds Using Artificial Intelligence for the Management of Allergic Diseases
- PMID: 38396957
- PMCID: PMC10889320
- DOI: 10.3390/ijms25042280
Identification of Hit Compounds Using Artificial Intelligence for the Management of Allergic Diseases
Abstract
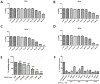
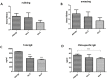
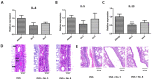
This study aimed to identify and evaluate drug candidates targeting the kinase inhibitory region of suppressor of cytokine signaling (SOCS) 3 for the treatment of allergic rhinitis (AR). Utilizing an artificial intelligence (AI)-based new drug development platform, virtual screening was conducted to identify compounds inhibiting the SH2 domain binding of SOCS3. Luminescence assays assessed the ability of these compounds to restore JAK-2 activity diminished by SOCS3. Jurkat T and BEAS-2B cells were utilized to investigate changes in SOCS3 and STAT3 expression, along with STAT3 phosphorylation in response to the identified compounds. In an OVA-induced allergic rhinitis mouse model, we measured serum levels of total IgE and OVA-specific IgE, performed real-time PCR on nasal mucosa samples to quantify Th2 cytokines and IFN-γ expression, and conducted immunohistochemistry to analyze eosinophil levels. Screening identified 20 hit compounds with robust binding affinities. As the concentration of SOCS3 increased, a corresponding decrease in JAK2 activity was observed. Compounds 5 and 8 exhibited significant efficacy in restoring JAK2 activity without toxicity. Treatment with these compounds resulted in reduced SOCS3 expression and the reinstatement of STAT3 phosphorylation in Jurkat T and BEAS-2B cells. In the OVA-induced allergic rhinitis mouse model, compounds 5 and 8 effectively alleviated nasal symptoms and demonstrated lower levels of immune markers compared to the allergy group. This study underscores the promising nonclinical efficacy of compounds identified through the AI-based drug development platform. These findings introduce innovative strategies for the treatment of AR and highlight the potential therapeutic value of targeting SOCS3 in managing AR.
Keywords: SOCS3; allergic rhinitis; artificial intelligence; new drug development platform.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Alpha-linolenic acid improves nasal mucosa epithelial barrier function in allergic rhinitis by arresting CD4+ T cell differentiation via IL-4Rα-JAK2-STAT3 pathway.Phytomedicine. 2023 Jul 25;116:154825. doi: 10.1016/j.phymed.2023.154825. Epub 2023 Apr 21. Phytomedicine. 2023. PMID: 37178572
-
Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced allergic rhinitis.Chem Biol Interact. 2020 Jan 5;315:108874. doi: 10.1016/j.cbi.2019.108874. Epub 2019 Oct 24. Chem Biol Interact. 2020. PMID: 31669322
-
Ameliorative effect of selective NLRP3 inflammasome inhibitor MCC950 in an ovalbumin-induced allergic rhinitis murine model.Int Immunopharmacol. 2020 Jun;83:106394. doi: 10.1016/j.intimp.2020.106394. Epub 2020 Mar 16. Int Immunopharmacol. 2020. PMID: 32193102
-
Aberrant expression of suppressor of cytokine signaling (SOCS) molecules contributes to the development of allergic diseases.Clin Exp Allergy. 2023 Nov;53(11):1147-1161. doi: 10.1111/cea.14385. Epub 2023 Aug 28. Clin Exp Allergy. 2023. PMID: 37641429 Review.
-
The potential application and molecular mechanisms of natural products in the treatment of allergic rhinitis: A review.Phytomedicine. 2024 Jul;129:155663. doi: 10.1016/j.phymed.2024.155663. Epub 2024 Apr 21. Phytomedicine. 2024. PMID: 38759345 Review.
Cited by
-
Application and research progress of artificial intelligence in allergic diseases.Int J Med Sci. 2025 Apr 9;22(9):2088-2102. doi: 10.7150/ijms.105422. eCollection 2025. Int J Med Sci. 2025. PMID: 40303497 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- NRF-2020R1A2C1006398/National Research Foundation of Korea
- 2017R1A2B2003575/National Research Foundation of Korea
- 2020R1C1C1012288/Ministry of Science and ICT
- IITP-2023-2020-0-01819/Institute for Information & communication Technology Planning & evaluation
- HI17C0387/Korea Health Industry Development Institute/Republic of Korea
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

