CSDE1 Intracellular Distribution as a Biomarker of Melanoma Prognosis
- PMID: 38396995
- PMCID: PMC10889260
- DOI: 10.3390/ijms25042319
CSDE1 Intracellular Distribution as a Biomarker of Melanoma Prognosis
Abstract
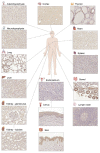
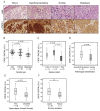
RNA-binding proteins are emerging as critical modulators of oncogenic cell transformation, malignancy and therapy resistance. We have previously found that the RNA-binding protein Cold Shock Domain containing protein E1 (CSDE1) promotes invasion and metastasis of melanoma, the deadliest form of skin cancer and also a highly heterogeneous disease in need of predictive biomarkers and druggable targets. Here, we design a monoclonal antibody useful for IHC in the clinical setting and use it to evaluate the prognosis potential of CSDE1 in an exploratory cohort of 149 whole tissue sections including benign nevi and primary tumors and metastasis from melanoma patients. Contrary to expectations for an oncoprotein, we observed a global decrease in CSDE1 levels with increasing malignancy. However, the CSDE1 cytoplasmic/nuclear ratio exhibited a positive correlation with adverse clinical features of primary tumors and emerged as a robust indicator of progression free survival in cutaneous melanoma, highlighting the potential of CSDE1 as a biomarker of prognosis. Our findings provide a novel feature for prognosis assessment and highlight the intricacies of RNA-binding protein dynamics in cancer progression.
Keywords: CSDE1; RNA-binding protein; biomarker; cytoplasmic–nuclear ratio; melanoma; prognosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- PGC2018-099697-B-I00/Ministerio de Ciencia e Innovación
- PID2021-127948NB-I00/Ministerio de Ciencia e Innovación
- ID 100010434 under grant LCF/PR/HR17/52150016/La Caixa Foundation
- SGR-Cat-2021-01215/Agency for Administration of University and Research
- CEX2020-001049-S, MCIN/AEI /10.13039/501100011033/Ministerio de Ciencia e Innovación
LinkOut - more resources
Full Text Sources
Medical

