The Impact of Psilocybin on High Glucose/Lipid-Induced Changes in INS-1 Cell Viability and Dedifferentiation
- PMID: 38397173
- PMCID: PMC10888174
- DOI: 10.3390/genes15020183
The Impact of Psilocybin on High Glucose/Lipid-Induced Changes in INS-1 Cell Viability and Dedifferentiation
Abstract
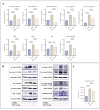
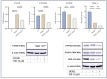
Serotonin emerges as a pivotal factor influencing the growth and functionality of β-cells. Psilocybin, a natural compound derived from mushrooms of the Psilocybe genus, exerts agonistic effects on the serotonin 5-HT2A and 5-HT2B receptors, thereby mimicking serotonin's behavior. This study investigates the potential impacts of psilocybin on β-cell viability, dedifferentiation, and function using an in vitro system. The INS-1 832/13 Rat Insulinoma cell line underwent psilocybin pretreatment, followed by exposure to high glucose-high lipid (HG-HL) conditions for specific time periods. After being harvested from treated cells, total transcript and cellular protein were utilized for further investigation. Our findings implied that psilocybin administration effectively mitigates HG-HL-stimulated β-cell loss, potentially mediated through the modulation of apoptotic biomarkers, which is possibly related to the mitigation of TXNIP, STAT-1, and STAT-3 phosphorylation. Furthermore, psilocybin exhibits the capacity to modulate the expression of key genes associated with β-cell dedifferentiation, including Pou5f1 and Nanog, indicating its potential in attenuating β-cell dedifferentiation. This research lays the groundwork for further exploration into the therapeutic potential of psilocybin in Type II diabetes intervention.
Keywords: diabetes; psilocybin; β-cell dedifferentiation; β-cell loss.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Holt R.I., Cockram C., Flyvbjerg A., Goldstein B.J. Textbook of Diabetes. John Wiley & Sons; Hoboken, NJ, USA: 2017.
-
- Amo-Shiinoki K., Tanabe K., Hoshii Y., Matsui H., Harano R., Fukuda T., Takeuchi T., Bouchi R., Takagi T., Hatanaka M. Islet cell dedifferentiation is a pathologic mechanism of long-standing progression of type 2 diabetes. JCI Insight. 2021;6:e143791. doi: 10.1172/jci.insight.143791. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

