Spitz Melanocytic Tumors: A Fascinating 75-Year Journey
- PMID: 38397186
- PMCID: PMC10887813
- DOI: 10.3390/genes15020195
Spitz Melanocytic Tumors: A Fascinating 75-Year Journey
Abstract
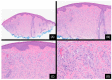
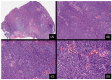
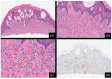
Over the last 75 years, our understanding of Spitz lesions has undergone substantial evolution. Initially considered a specific type of melanoma, the perception has shifted towards recognizing Spitz lesions as a spectrum comprising Spitz nevi, Spitz melanocytomas, and Spitz melanomas. Spitz lesions are known for posing a significant diagnostic challenge regarding the distinction between benign neoplasms displaying atypical traits and melanomas. A comprehensive understanding of their molecular basis and genomic aberrations has significantly improved precision in classifying and diagnosing these challenging lesions. The primary aim of this review is to encapsulate the current understanding of the molecular pathogenesis and distinct clinicopathologic characteristics defining this intriguing set of tumors.
Keywords: atypical spitz tumor; melanocytic; melanoma; nevus; spitz; spitzoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Heymann W.R. Sophie’s Choice: Assessing Atypical Spitz Tumors in the Molecular Era. SKINmed. 2020;18:27–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

