Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology
- PMID: 38397377
- PMCID: PMC10886568
- DOI: 10.3390/biom14020140
Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology
Abstract
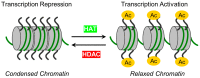
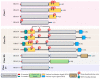
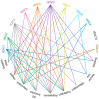
A monolayer of endothelial cells (ECs) lines the lumen of blood vessels and, as such, provides a semi-selective barrier between the blood and the interstitial space. Compromise of the lung EC barrier due to inflammatory or toxic events may result in pulmonary edema, which is a cardinal feature of acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS). The EC functions are controlled, at least in part, via epigenetic mechanisms mediated by histone deacetylases (HDACs). Zinc-dependent HDACs represent the largest group of HDACs and are activated by Zn2+. Members of this HDAC group are involved in epigenetic regulation primarily by modifying the structure of chromatin upon removal of acetyl groups from histones. In addition, they can deacetylate many non-histone histone proteins, including those located in extranuclear compartments. Recently, the therapeutic potential of inhibiting zinc-dependent HDACs for EC barrier preservation has gained momentum. However, the role of specific HDAC subtypes in EC barrier regulation remains largely unknown. This review aims to provide an update on the role of zinc-dependent HDACs in endothelial dysfunction and its related diseases. We will broadly focus on biological contributions, signaling pathways and transcriptional roles of HDACs in endothelial pathobiology associated mainly with lung diseases, and we will discuss the potential of their inhibitors for lung injury prevention.
Keywords: HDAC inhibitors; acute lung injury; acute respiratory distress syndrome; deacetylation; endothelial barrier integrity; lung vascular endothelium; zinc-dependent HDACs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

