Alzheimer's Amyloid-β Accelerates Cell Senescence and Suppresses SIRT1 in Human Neural Stem Cells
- PMID: 38397428
- PMCID: PMC10886734
- DOI: 10.3390/biom14020189
Alzheimer's Amyloid-β Accelerates Cell Senescence and Suppresses SIRT1 in Human Neural Stem Cells
Abstract
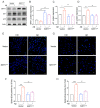
As a lifelong source of neurons, neural stem cells (NSCs) serve multiple crucial functions in the brain. The senescence of NSCs may be associated with the onset and progression of Alzheimer's disease (AD). Our study reveals a noteworthy finding, indicating that the AD-associated pathogenic protein amyloid-β (Aβ) substantially enhances senescence-related characteristics of human NSCs. These characteristics encompass the enhanced expression of p16 and p21, the upregulation of genes associated with the senescence-associated secretory phenotype (SASP), increased SA-β-gal activity, and the activation of the DNA damage response. Further studies revealed that Aβ treatment significantly downregulates the SIRT1 protein which plays a crucial role in regulating the aging process and decreases downstream PGC-1α and FOXO3. Subsequently, we found that SIRT1 overexpression significantly alleviates a range of Aβ-induced senescent markers in human NSCs. Taken together, our results uncover that Aβ accelerates cellular senescence in human NSCs, making SIRT1 a highly promising therapeutic target for senescent NSCs which may contribute to age-related neurodegenerative diseases, including AD.
Keywords: Aβ; ROS; SIRT1; cell senescence; neural stem cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

