Discovery of A Novel Series of Quinazoline-Thiazole Hybrids as Potential Antiproliferative and Anti-Angiogenic Agents
- PMID: 38397456
- PMCID: PMC10886515
- DOI: 10.3390/biom14020218
Discovery of A Novel Series of Quinazoline-Thiazole Hybrids as Potential Antiproliferative and Anti-Angiogenic Agents
Abstract
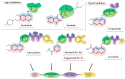
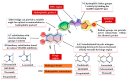
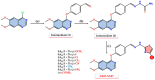
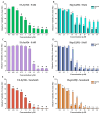
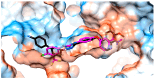
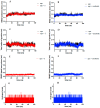
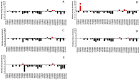
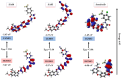
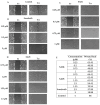
Considering the pivotal role of angiogenesis in solid tumor progression, we developed a novel series of quinazoline-thiazole hybrids (SA01-SA07) as antiproliferative and anti-angiogenic agents. Four out of the seven compounds displayed superior antiproliferative activity (IC50 =1.83-4.24 µM) on HepG2 cells compared to sorafenib (IC50 = 6.28 µM). The affinity towards the VEGFR2 kinase domain was assessed through in silico prediction by molecular docking, molecular dynamics studies, and MM-PBSA. The series displayed a high degree of similarity to sorafenib regarding the binding pose within the active site of VEGFR2, with a different orientation of the 4-substituted-thiazole moieties in the allosteric pocket. Molecular dynamics and MM-PBSA evaluations identified SA05 as the hybrid forming the most stable complex with VEGFR2 compared to sorafenib. The impact of the compounds on vascular cell proliferation was assessed on EA.hy926 cells. Six compounds (SA01-SA05, SA07) displayed superior anti-proliferative activity (IC50 = 0.79-5.85 µM) compared to sorafenib (IC50 = 6.62 µM). The toxicity was evaluated on BJ cells. Further studies of the anti-angiogenic effect of the most promising compounds, SA04 and SA05, through the assessment of impact on EA.hy296 motility using a wound healing assay and in ovo potential in a CAM assay compared to sorafenib, led to the confirmation of the anti-angiogenic potential.
Keywords: VEGFR2; anti-angiogenic; antiproliferative; cancer; drug discovery; hybrid compounds; quinazoline; small molecules; structure design; synthesis; thiazole; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rational Design and Synthesis of a Novel Series of Thiosemicarbazone-Containing Quinazoline Derivatives as Potential VEGFR2 Inhibitors.Pharmaceutics. 2025 Feb 15;17(2):260. doi: 10.3390/pharmaceutics17020260. Pharmaceutics. 2025. PMID: 40006627 Free PMC article.
-
Design and synthesis of some new 6-bromo-2-(pyridin-3-yl)-4-substituted quinazolines as multi tyrosine kinase inhibitors.Bioorg Chem. 2022 Nov;128:106099. doi: 10.1016/j.bioorg.2022.106099. Epub 2022 Aug 17. Bioorg Chem. 2022. PMID: 35994884
-
Discovery of new quinazolin-4(3H)-ones as VEGFR-2 inhibitors: Design, synthesis, and anti-proliferative evaluation.Bioorg Chem. 2020 Dec;105:104380. doi: 10.1016/j.bioorg.2020.104380. Epub 2020 Oct 15. Bioorg Chem. 2020. PMID: 33128967
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
-
Quinazoline-based VEGFR-2 inhibitors as potential anti-angiogenic agents: A contemporary perspective of SAR and molecular docking studies.Eur J Med Chem. 2023 Nov 5;259:115626. doi: 10.1016/j.ejmech.2023.115626. Epub 2023 Jul 8. Eur J Med Chem. 2023. PMID: 37453330 Review.
Cited by
-
Recent studies on protein kinase signaling inhibitors based on thiazoles: review to date.RSC Adv. 2024 Nov 19;14(50):36989-37018. doi: 10.1039/d4ra05601a. eCollection 2024 Nov 19. RSC Adv. 2024. PMID: 39569127 Free PMC article. Review.
-
Synthesis, Cytotoxicity and Antioxidant Activity Evaluation of Some Thiazolyl-Catechol Compounds.Antioxidants (Basel). 2024 Aug 1;13(8):937. doi: 10.3390/antiox13080937. Antioxidants (Basel). 2024. PMID: 39199183 Free PMC article.
-
Rational Design and Synthesis of a Novel Series of Thiosemicarbazone-Containing Quinazoline Derivatives as Potential VEGFR2 Inhibitors.Pharmaceutics. 2025 Feb 15;17(2):260. doi: 10.3390/pharmaceutics17020260. Pharmaceutics. 2025. PMID: 40006627 Free PMC article.
-
Quinazolinones as Potential Anticancer Agents: Synthesis and Action Mechanisms.Biomolecules. 2025 Feb 1;15(2):210. doi: 10.3390/biom15020210. Biomolecules. 2025. PMID: 40001513 Free PMC article. Review.
References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

