Comprehensive Analysis of the Role of Fibrinogen and Thrombin in Clot Formation and Structure for Plasma and Purified Fibrinogen
- PMID: 38397467
- PMCID: PMC10886591
- DOI: 10.3390/biom14020230
Comprehensive Analysis of the Role of Fibrinogen and Thrombin in Clot Formation and Structure for Plasma and Purified Fibrinogen
Abstract
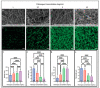
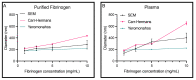
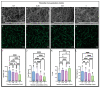
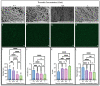
Altered properties of fibrin clots have been associated with bleeding and thrombotic disorders, including hemophilia or trauma and heart attack or stroke. Clotting factors, such as thrombin and tissue factor, or blood plasma proteins, such as fibrinogen, play critical roles in fibrin network polymerization. The concentrations and combinations of these proteins affect the structure and stability of clots, which can lead to downstream complications. The present work includes clots made from plasma and purified fibrinogen and shows how varying fibrinogen and activation factor concentrations affect the fibrin properties under both conditions. We used a combination of scanning electron microscopy, confocal microscopy, and turbidimetry to analyze clot/fiber structure and polymerization. We quantified the structural and polymerization features and found similar trends with increasing/decreasing fibrinogen and thrombin concentrations for both purified fibrinogen and plasma clots. Using our compiled results, we were able to generate multiple linear regressions that predict structural and polymerization features using various fibrinogen and clotting agent concentrations. This study provides an analysis of structural and polymerization features of clots made with purified fibrinogen or plasma at various fibrinogen and clotting agent concentrations. Our results could be utilized to aid in interpreting results, designing future experiments, or developing relevant mathematical models.
Keywords: fibrin; fibrinogen; thrombin; thrombosis; tissue factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Tomaiuolo M., Brass L.F. Joining forces to understand hemostasis and thrombosis: A call to communicate. Comment on” Modeling thrombosis in silico: Frontiers, challenges, unresolved problems and milestones” by AV Belyaev et al. Phys. Life Rev. 2018;26:110–112. doi: 10.1016/j.plrev.2018.06.019. - DOI - PubMed
-
- Domingues M.M., Macrae F.L., Duval C., McPherson H.R., Bridge K.I., Ajjan R.A., Ridger V.C., Connell S.D., Philippou H., Ariëns R.A. Thrombin and fibrinogen γ′ impact clot structure by marked effects on intrafibrillar structure and protofibril packing. Blood J. Am. Soc. Hematol. 2016;127:487–495. doi: 10.1182/blood-2015-06-652214. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

