Liposomal Glutathione Augments Immune Defenses against Respiratory Syncytial Virus in Neonatal Mice Exposed in Utero to Ethanol
- PMID: 38397736
- PMCID: PMC10886408
- DOI: 10.3390/antiox13020137
Liposomal Glutathione Augments Immune Defenses against Respiratory Syncytial Virus in Neonatal Mice Exposed in Utero to Ethanol
Abstract
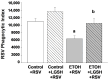
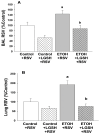
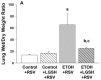
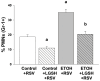
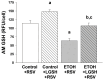
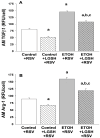
We previously reported that maternal alcohol use increased the risk of sepsis in premature and term newborns. In the neonatal mouse, fetal ethanol (ETOH) exposure depleted the antioxidant glutathione (GSH), which promoted alveolar macrophage (AM) immunosuppression and respiratory syncytial virus (RSV) infections. In this study, we explored if oral liposomal GSH (LGSH) would attenuate oxidant stress and RSV infections in the ETOH-exposed mouse pups. C57BL/6 female mice were pair-fed a liquid diet with 25% of calories from ethanol or maltose-dextrin. Postnatal day 10 pups were randomized to intranasal saline, LGSH, and RSV. After 48 h, we assessed oxidant stress, AM immunosuppression, pulmonary RSV burden, and acute lung injury. Fetal ETOH exposure increased oxidant stress threefold, lung RSV burden twofold and acute lung injury threefold. AMs were immunosuppressed with decreased RSV clearance. However, LGSH treatments of the ETOH group normalized oxidant stress, AM immune phenotype, the RSV burden, and acute lung injury. These studies suggest that the oxidant stress caused by fetal ETOH exposure impaired AM clearance of infectious agents, thereby increasing the viral infection and acute lung injury. LGSH treatments reversed the oxidative stress and restored AM immune functions, which decreased the RSV infection and subsequent acute lung injury.
Keywords: alveolar macrophage; fetal ethanol exposure; liposomal glutathione; oxidant stress; respiratory syncytial virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of zinc insufficiency in fetal alveolar macrophage dysfunction and RSV exacerbation associated with fetal ethanol exposure.Alcohol. 2019 Nov;80:5-16. doi: 10.1016/j.alcohol.2018.11.007. Epub 2018 Dec 21. Alcohol. 2019. PMID: 30580016 Free PMC article.
-
Respiratory syncytial virus vaccination during pregnancy for improving infant outcomes.Cochrane Database Syst Rev. 2024 May 2;5(5):CD015134. doi: 10.1002/14651858.CD015134.pub2. Cochrane Database Syst Rev. 2024. PMID: 38695784 Free PMC article.
-
Sexual dimorphism in lung transcriptomic adaptations in fetal alcohol spectrum disorders.Respir Res. 2025 Jan 8;26(1):6. doi: 10.1186/s12931-025-03094-z. Respir Res. 2025. PMID: 39780208 Free PMC article.
-
Elimination of gut microbiota hinders the therapeutic effect of amentoflavone on respiratory syncytial virus-induced lung inflammation injury by regulating innate immunity.Phytomedicine. 2025 Sep;145:157033. doi: 10.1016/j.phymed.2025.157033. Epub 2025 Jul 8. Phytomedicine. 2025. PMID: 40701130
-
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD009417. doi: 10.1002/14651858.CD009417.pub3. Cochrane Database Syst Rev. 2023. PMID: 37870128 Free PMC article.
Cited by
-
Alcohol in Daily Products: Health Risks, Cultural Considerations, and Economic Impacts.Risk Manag Healthc Policy. 2025 Jan 18;18:217-237. doi: 10.2147/RMHP.S495493. eCollection 2025. Risk Manag Healthc Policy. 2025. PMID: 39845405 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

