Mechanism of Antiradical Activity of Coumarin-Trihydroxybenzohydrazide Derivatives: A Comprehensive Kinetic DFT Study
- PMID: 38397741
- PMCID: PMC10885972
- DOI: 10.3390/antiox13020143
Mechanism of Antiradical Activity of Coumarin-Trihydroxybenzohydrazide Derivatives: A Comprehensive Kinetic DFT Study
Abstract
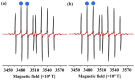
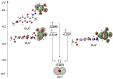
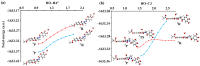
As part of this study, the mechanisms of the antioxidant activity of previously synthesized coumarin-trihydrobenzohydrazine derivatives were investigated: (E)-2,4-dioxo-3-(1-(2-(2″,3″,4″-trihydroxybenzoyl)hydrazineyl)ethylidene)chroman-7-yl acetate (1) and (E)-2,4-dioxo-3-(1-(2-(3″,4″,5″-trihydroxybenzoyl)hydrazineyl)ethylidene)chroman-7-yl acetate (2). The capacity of the compounds to neutralize HO• was assessed by EPR spectroscopy. The standard mechanisms of antioxidant action, Hydrogen Atom Transfer (HAT), Sequential Proton Loss followed by Electron Transfer (SPLET), Single-Electron Transfer followed by Proton Transfer (SET-PT), and Radical Adduct/Coupling Formation (RAF/RCF) were examined using the QM-ORSA methodology. It was estimated that the newly synthesized compounds, under physiological conditions, exhibited antiradical activity via SPLET and RCF mechanisms. Based on the estimated overall rate constants (koverall), it can be concluded that 2 exhibited a greater antiradical capacity. The obtained values indicated a good correlation with the EPR spectroscopy results. Both compounds exhibit approximately 1.5 times more activity in comparison to the precursor compound used in the synthesis (gallic acid).
Keywords: QM-ORSA; antioxidant; coumarin; free radicals; gallic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

