Anti-Inflammatory Effects of Idebenone Attenuate LPS-Induced Systemic Inflammatory Diseases by Suppressing NF-κB Activation
- PMID: 38397749
- PMCID: PMC10885939
- DOI: 10.3390/antiox13020151
Anti-Inflammatory Effects of Idebenone Attenuate LPS-Induced Systemic Inflammatory Diseases by Suppressing NF-κB Activation
Abstract
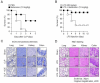
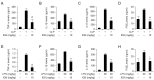
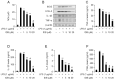
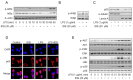
Inflammation is a natural protective process through which the immune system responds to injury, infection, or irritation. However, hyperinflammation or long-term inflammatory responses can cause various inflammatory diseases. Although idebenone was initially developed for the treatment of cognitive impairment and dementia, it is currently used to treat various diseases. However, its anti-inflammatory effects and regulatory functions in inflammatory diseases are yet to be elucidated. Therefore, this study aimed to investigate the anti-inflammatory effects of idebenone in cecal ligation puncture-induced sepsis and lipopolysaccharide-induced systemic inflammation. Murine models of cecal ligation puncture-induced sepsis and lipopolysaccharide-induced systemic inflammation were generated, followed by treatment with various concentrations of idebenone. Additionally, lipopolysaccharide-stimulated macrophages were treated with idebenone to elucidate its anti-inflammatory effects at the cellular level. Idebenone treatment significantly improved survival rate, protected against tissue damage, and decreased the expression of inflammatory enzymes and cytokines in mice models of sepsis and systemic inflammation. Additionally, idebenone treatment suppressed inflammatory responses in macrophages, inhibited the NF-κB signaling pathway, reduced reactive oxygen species and lipid peroxidation, and normalized the activities of antioxidant enzyme. Idebenone possesses potential therapeutic application as a novel anti-inflammatory agent in systemic inflammatory diseases and sepsis.
Keywords: NF-κB; ROS; anti-inflammation; idebenone; inflammatory disease model.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Horiguchi H., Loftus T.J., Hawkins R.B., Raymond S.L., Stortz J.A., Hollen M.K., Weiss B.P., Miller E.S., Bihorac A., Larson S.D. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front. Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

