Ascorbic Acid Protects Bone Marrow from Oxidative Stress and Transient Elevation of Corticosterone Caused by X-ray Exposure in Akr1a-Knockout Mice
- PMID: 38397750
- PMCID: PMC10886414
- DOI: 10.3390/antiox13020152
Ascorbic Acid Protects Bone Marrow from Oxidative Stress and Transient Elevation of Corticosterone Caused by X-ray Exposure in Akr1a-Knockout Mice
Abstract
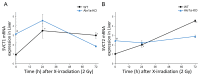
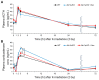
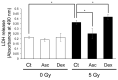
Bone marrow cells are the most sensitive to exposure to X-rays in the body and are selectively damaged even by doses that are generally considered permissive in other organs. Ascorbic acid (Asc) is a potent antioxidant that is reported to alleviate damages caused by X-ray exposure. However, rodents can synthesize Asc, which creates difficulties in rigorously assessing its effects in such laboratory animals. To address this issue, we employed mice with defects in their ability to synthesize Asc due to a genetic ablation of aldehyde reductase (Akr1a-KO). In this study, concentrations of white blood cells (WBCs) were decreased 3 days after exposure to X-rays at 2 Gy and then gradually recovered. At approximately one month, the recovery rate of WBCs was delayed in the Akr1a-KO mouse group, which was reversed via supplementation with Asc. Following exposure to X-rays, Asc levels decreased in plasma, bone marrow cells, and the liver during an early period, and then started to increase. X-ray exposure stimulated the pituitary gland to release adrenocorticotropic hormone (ACTH), which stimulated corticosterone secretion. Asc released from the liver, which was also stimulated by ACTH, appeared to be recruited to the bone marrow. Since corticosterone in high doses is injurious, these collective results imply that Asc protects bone marrow via its antioxidant capacity against ROS produced via exposure to X-rays and the cytotoxic action of transiently elevated corticosterone.
Keywords: adrenal; adrenocorticotropic hormone; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Developmental retardation in neonates of aldehyde reductase (AKR1A)-deficient mice is associated with low ascorbic acid and high corticosterone levels.J Nutr Biochem. 2021 May;91:108604. doi: 10.1016/j.jnutbio.2021.108604. Epub 2021 Feb 4. J Nutr Biochem. 2021. PMID: 33549889
-
Ablation of aldehyde reductase aggravates carbon tetrachloride-induced acute hepatic injury involving oxidative stress and endoplasmic reticulum stress.Biochem Biophys Res Commun. 2016 Sep 16;478(2):765-71. doi: 10.1016/j.bbrc.2016.08.022. Epub 2016 Aug 5. Biochem Biophys Res Commun. 2016. PMID: 27501753
-
Ascorbic acid prevents N-nitrosodiethylamine-induced hepatic injury and hepatocarcinogenesis in Akr1a-knockout mice.Toxicol Lett. 2020 Oct 15;333:192-201. doi: 10.1016/j.toxlet.2020.08.005. Epub 2020 Aug 14. Toxicol Lett. 2020. PMID: 32805337
-
Genetic ablation of aldehyde reductase (Akr1a) augments exercise endurance in mice via activation of the PGC-1α-involved pathway.Life Sci. 2020 May 15;249:117501. doi: 10.1016/j.lfs.2020.117501. Epub 2020 Mar 3. Life Sci. 2020. PMID: 32142766
-
Ascorbic acid reverses the prolonged anesthetic action of pentobarbital in Akr1a-knockout mice.Life Sci. 2014 Jan 24;95(1):1-8. doi: 10.1016/j.lfs.2013.12.004. Epub 2013 Dec 17. Life Sci. 2014. PMID: 24355294
Cited by
-
Role of oxidative stress in impaired type II diabetic bone repair: scope for antioxidant therapy intervention?Front Dent Med. 2024 Oct 14;5:1464009. doi: 10.3389/fdmed.2024.1464009. eCollection 2024. Front Dent Med. 2024. PMID: 39917650 Free PMC article. Review.
References
-
- Orschell C.M., Wu T., Patterson A.M. Impact of Age, Sex, and Genetic Diversity in Murine Models of the Hematopoietic Acute Radiation Syndrome (H-ARS) and the Delayed Effects of Acute Radiation Exposure (DEARE) Curr. Stem Cell Rep. 2022;8:139–149. doi: 10.1007/s40778-022-00214-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

