SARS-CoV-2 Spike Protein Stimulates Macropinocytosis in Murine and Human Macrophages via PKC-NADPH Oxidase Signaling
- PMID: 38397773
- PMCID: PMC10885885
- DOI: 10.3390/antiox13020175
SARS-CoV-2 Spike Protein Stimulates Macropinocytosis in Murine and Human Macrophages via PKC-NADPH Oxidase Signaling
Abstract
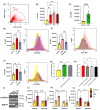
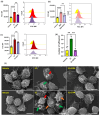
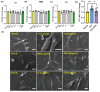
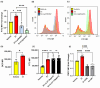
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While recent studies have demonstrated that SARS-CoV-2 may enter kidney and colon epithelial cells by inducing receptor-independent macropinocytosis, it remains unknown whether this process also occurs in cell types directly relevant to SARS-CoV-2-associated lung pneumonia, such as alveolar epithelial cells and macrophages. The goal of our study was to investigate the ability of SARS-CoV-2 spike protein subunits to stimulate macropinocytosis in human alveolar epithelial cells and primary human and murine macrophages. Flow cytometry analysis of fluid-phase marker internalization demonstrated that SARS-CoV-2 spike protein subunits S1, the receptor-binding domain (RBD) of S1, and S2 stimulate macropinocytosis in both human and murine macrophages in an angiotensin-converting enzyme 2 (ACE2)-independent manner. Pharmacological and genetic inhibition of macropinocytosis substantially decreased spike-protein-induced fluid-phase marker internalization in macrophages both in vitro and in vivo. High-resolution scanning electron microscopy (SEM) imaging confirmed that spike protein subunits promote the formation of membrane ruffles on the dorsal surface of macrophages. Mechanistic studies demonstrated that SARS-CoV-2 spike protein stimulated macropinocytosis via NADPH oxidase 2 (Nox2)-derived reactive oxygen species (ROS) generation. In addition, inhibition of protein kinase C (PKC) and phosphoinositide 3-kinase (PI3K) in macrophages blocked SARS-CoV-2 spike-protein-induced macropinocytosis. To our knowledge, these results demonstrate for the first time that SARS-CoV-2 spike protein subunits stimulate macropinocytosis in macrophages. These results may contribute to a better understanding of SARS-CoV-2 infection and COVID-19 pathogenesis.
Keywords: SARS-CoV-2; epithelial cell; macrophage; macropinocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
An engineered nano-liposome-human ACE2 decoy neutralizes SARS-CoV-2 Spike protein-induced inflammation in both murine and human macrophages.Theranostics. 2022 Mar 6;12(6):2639-2657. doi: 10.7150/thno.66831. eCollection 2022. Theranostics. 2022. PMID: 35401811 Free PMC article.
-
SARS-CoV-2 hijacks macropinocytosis to facilitate its entry and promote viral spike-mediated cell-to-cell fusion.J Biol Chem. 2022 Nov;298(11):102511. doi: 10.1016/j.jbc.2022.102511. Epub 2022 Sep 19. J Biol Chem. 2022. PMID: 36259516 Free PMC article.
-
Nox2-Mediated PI3K and Cofilin Activation Confers Alternate Redox Control of Macrophage Pinocytosis.Antioxid Redox Signal. 2017 Jun 1;26(16):902-916. doi: 10.1089/ars.2016.6639. Epub 2016 Sep 13. Antioxid Redox Signal. 2017. PMID: 27488058 Free PMC article.
-
Interactions of angiotensin-converting enzyme-2 (ACE2) and SARS-CoV-2 spike receptor-binding domain (RBD): a structural perspective.Mol Biol Rep. 2023 Mar;50(3):2713-2721. doi: 10.1007/s11033-022-08193-4. Epub 2022 Dec 23. Mol Biol Rep. 2023. PMID: 36562937 Free PMC article. Review.
-
Insights on SARS-CoV-2 Molecular Interactions With the Renin-Angiotensin System.Front Cell Dev Biol. 2020 Sep 16;8:559841. doi: 10.3389/fcell.2020.559841. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33042994 Free PMC article. Review.
References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

