Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer's Disease Inflammation
- PMID: 38397775
- PMCID: PMC10886200
- DOI: 10.3390/antiox13020177
Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer's Disease Inflammation
Abstract
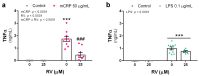
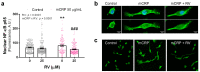
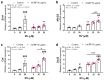
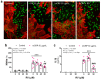
Resveratrol is a natural phenolic compound with known benefits against neurodegeneration. We analyzed in vitro the protective mechanisms of resveratrol against the proinflammatory monomeric C-reactive protein (mCRP). mCRP increases the risk of AD after stroke and we previously demonstrated that intracerebral mCRP induces AD-like dementia in mice. Here, we used BV2 microglia treated with mCRP for 24 h in the presence or absence of resveratrol. Cells and conditioned media were collected for analysis. Lipopolysaccharide (LPS) has also been implicated in AD progression and so LPS was used as a resveratrol-sensitive reference agent. mCRP at the concentration of 50 µg/mL activated the nitric oxide pathway and the NLRP3 inflammasome pathway. Furthermore, mCRP induced cyclooxygenase-2 and the release of proinflammatory cytokines. Resveratrol effectively inhibited these changes and increased the expression of the antioxidant enzyme genes Cat and Sod2. As central mechanisms of defense, resveratrol activated the hub genes Sirt1 and Nfe2l2 and inhibited the nuclear translocation of the signal transducer NF-ĸB. Proinflammatory changes induced by mCRP in primary mixed glial cultures were also protected by resveratrol. This work provides a mechanistic insight into the protective benefits of resveratrol in preventing the risk of AD induced by proinflammatory agents.
Keywords: BV2; inflammation; lipopolysaccharide; monomeric C-reactive protein; oxidative stress; primary mixed glial cultures; resveratrol.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
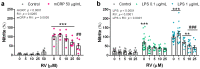



References
-
- Gupta C., Sharma G., Chan D. Phytochemicals of Nutraceutical Importance. CABI; Wallingford, UK: 2014. Resveratrol: A chemo-preventative agent with diverse applications; pp. 47–60. - DOI
-
- Grinan-Ferre C., Bellver-Sanchis A., Izquierdo V., Corpas R., Roig-Soriano J., Chillón M., Andres-Lacueva C., Somogyvári M., Sőti C., Sanfeliu C., et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021;67:101271. doi: 10.1016/j.arr.2021.101271. - DOI - PubMed
-
- Menet M.C., Baron S., Taghi M., Diestra R., Dargère D., Laprévote O., Nivet-Antoine V., Beaudeux J.L., Bédarida T., Cottart C.H. Distribution of trans-resveratrol and its metabolites after acute or sustained administration in mouse heart, brain, and liver. Mol. Nutr. Food Res. 2017;61:1600686. doi: 10.1002/mnfr.201600686. - DOI - PubMed
Grants and funding
- MICIN/AEI/10.13039/501100011033, PDC2021-121096-C22/Ministerio de Ciencia e Innovación
- MICIN/AEI/10.13039/501100011033, PID2019-106285-C22/Ministerio de Ciencia e Innovación
- 2021-SGR-357/Agency for Administration of University and Research
- nr. PCE 60/2021, cod proiect: PN-III-P4-ID-PCE-2020-1540/CREDICARD
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

