Determination of Nitric Oxide and Its Metabolites in Biological Tissues Using Ozone-Based Chemiluminescence Detection: A State-of-the-Art Review
- PMID: 38397777
- PMCID: PMC10886078
- DOI: 10.3390/antiox13020179
Determination of Nitric Oxide and Its Metabolites in Biological Tissues Using Ozone-Based Chemiluminescence Detection: A State-of-the-Art Review
Abstract
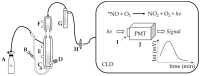
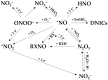
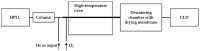
Ozone-based chemiluminescence detection (CLD) has been widely applied for determining nitric oxide (•NO) and its derived species in many different fields, such as environmental monitoring and biomedical research. In humans and animals, CLD has been applied to determine exhaled •NO and •NO metabolites in plasma and tissues. The main advantages of CLD are high sensitivity and selectivity for quantitative analysis in a wide dynamic range. Combining CLD with analytical separation techniques like chromatography allows for the analytes to be quantified with less disturbance from matrix components or impurities. Sampling techniques like microdialysis and flow injection analysis may be coupled to CLD with the possibility of real-time monitoring of •NO. However, details and precautions in experimental practice need to be addressed and clarified to avoid wrong estimations. Therefore, using CLD as a detection tool requires a deep understanding of the sample preparation procedure and chemical reactions used for liberating •NO from its derived species. In this review, we discuss the advantages and pitfalls of CLD for determining •NO species, list the different applications and combinations with other analytical techniques, and provide general practical notes for sample preparation. These guidelines are designed to assist researchers in comprehending CLD data and in selecting the most appropriate method for measuring •NO species.
Keywords: chemiluminescence detection; clinical studies; vascular function; •NO metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
[Application progress of on-line sample preparation techniques coupled with liquid chromatography-mass spectrometry system in the detection of food hazards].Se Pu. 2023 Dec;41(12):1062-1072. doi: 10.3724/SP.J.1123.2023.04026. Se Pu. 2023. PMID: 38093536 Free PMC article. Chinese.
-
Direct measurement of nitric oxide (NO) production rates from enzymes using ozone-based gas-phase chemiluminescence (CL).Nitric Oxide. 2021 Dec 1;117:60-71. doi: 10.1016/j.niox.2021.10.001. Epub 2021 Oct 13. Nitric Oxide. 2021. PMID: 34653611
-
Chemiluminescence-based Assays for Detection of Nitric Oxide and its Derivatives from Autoxidation and Nitrosated Compounds.J Vis Exp. 2022 Feb 16;(180). doi: 10.3791/63107. J Vis Exp. 2022. PMID: 35253799
-
Analytical applications of flow injection with chemiluminescence detection--a review.Luminescence. 2001 Jan-Feb;16(1):1-23. doi: 10.1002/bio.607. Luminescence. 2001. PMID: 11180653 Review.
-
Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 May 15;851(1-2):93-105. doi: 10.1016/j.jchromb.2006.12.012. Epub 2007 Jan 5. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17208057 Review.
Cited by
-
Current clinical framework on nitric oxide role in periodontal disease and blood pressure.Clin Oral Investig. 2024 Sep 12;28(10):521. doi: 10.1007/s00784-024-05913-x. Clin Oral Investig. 2024. PMID: 39264471 Free PMC article. Review.
-
OzNOxESI: A Novel Mass Spectrometry Ion Chemistry for Elucidating Lipid Double-Bond Regioisomerism in Complex Mixtures.Anal Chem. 2025 Jan 28;97(3):1879-1888. doi: 10.1021/acs.analchem.4c05940. Epub 2025 Jan 16. Anal Chem. 2025. PMID: 39817428 Free PMC article.
References
-
- Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. - DOI - PMC - PubMed
-
- Albrecht H.O. Über die Chemiluminescenz des Aminophthalsäurehydrazids. Z. Für Phys. Chem. 1928;136U:321–330. doi: 10.1515/zpch-1928-13625. - DOI
-
- Whitehead T.P., Thorpe G.H.G., Carter T.J.N., Groucutt C., Kricka L.J. Enhanced luminescence procedure for sensitive determination of peroxidase-labelled conjugates in immunoassay. Nature. 1983;305:158–159. doi: 10.1038/305158a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

